- Fermented sweet sorghum residue has higher adsorption capacity for Cr(VI) than original one.
- The maximum adsorption capacity can reach 16.39 mg/g (at 303.15 K).
- XANES characterizes interaction between fermented straw residue and Cr ions.
- The adsorption mechanism is mainly due to reduction reaction and chemical adsorption.
- Open Access
- Article
Biosorption of Chromium (VI) in Aqueous Solution by Fermented Sweet Sorghum Residues: Kinetics, Isotherm and Mechanism
- Jinling Wu 1,2,*,
- Jing Dong 1,
- Xuan Guo 1
Author Information
Received: 21 Nov 2025 | Revised: 15 Dec 2025 | Accepted: 30 Dec 2025 | Published: 08 Jan 2026
Highlights
Abstract
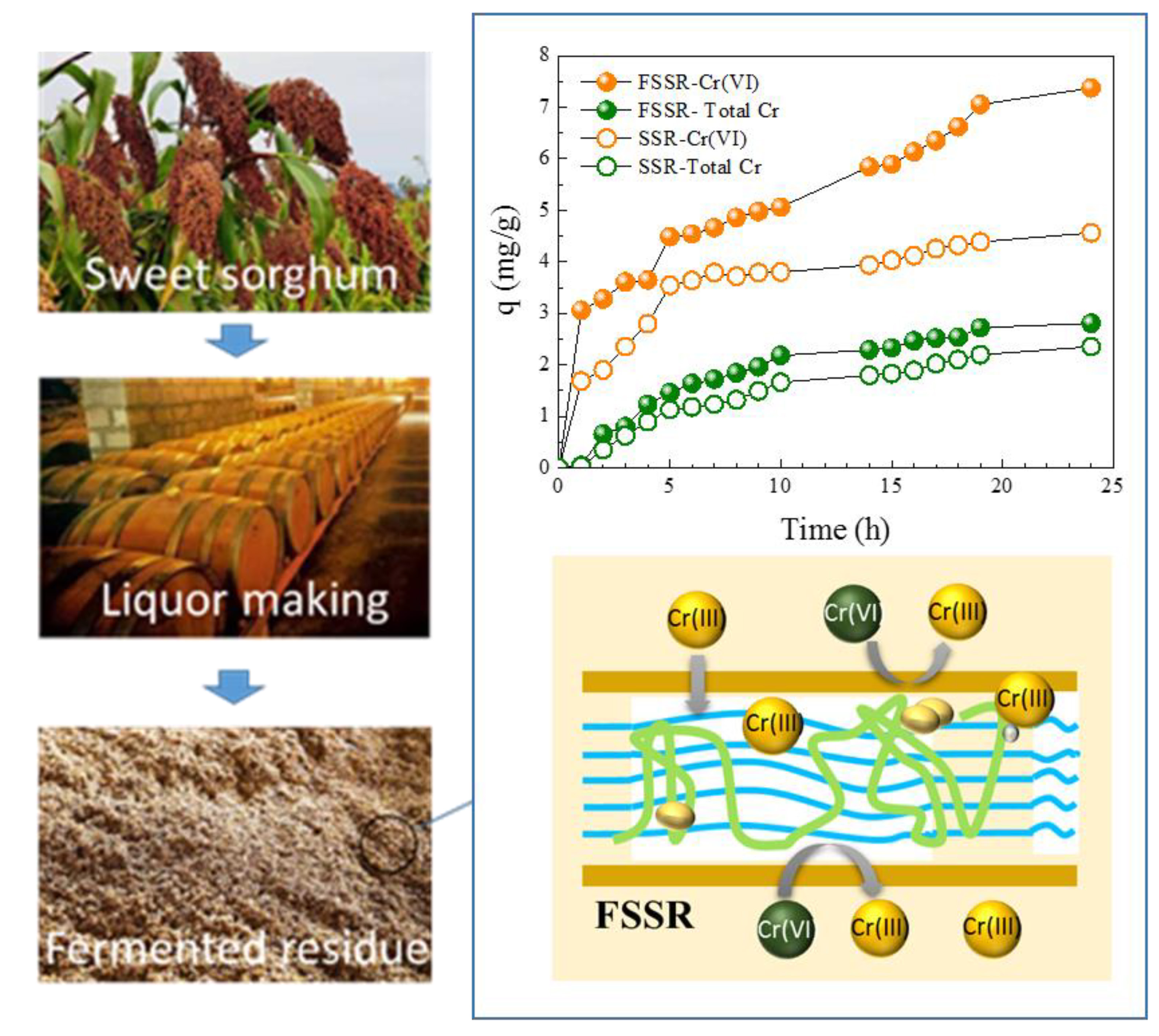
Fermented sweet sorghum residues (FSSR) were developed as an eco-friendly and low-cost biosorbent for the removal of chromium(Cr)(VI) from aqueous solutions, and their adsorption kinetics, isotherm and mechanism, and practical application potential were systematically investigated. The results showed that under the conditions of an initial Cr(VI) concentration of 50 mg/L, pH = 2.0, adsorbent dosage of 0.1 g, and temperature of 303 K, the equilibrium adsorption capacity (qe) of FSSR for Cr(VI) reached 7.37 ± 0.44 mg/g, which was 61.6% higher than that of original sweet sorghum residues (SSR, 4.56 ± 0.19 mg/g); an independent-samples t-test confirmed that the difference was statistically significant (p < 0.05). Kinetic analysis revealed that Cr(VI) adsorption by FSSR followed the pseudo-second-order model (R2 > 0.91; k2 = 0.021–0.079 g/(mg·h)) and the Elovich model (R2 > 0.95; α = 0.306–0.502 mg/(g·h), β = 0.625–1.010 g/mg), indicating that chemisorption dominated the adsorption process, while equilibrium data were best described by the Freundlich isotherm model (R2 > 0.95, KF = 0.83–4.76 mg/g, n = 2.01–3.64), suggesting heterogeneous multi-layer adsorption. Advanced characterization techniques, including X-ray adsorption near-edge structure (XANES) and energy-dispersive spectroscopy (EDS), demonstrated a dual removal mechanism in which Cr(VI) was first reduced to Cr(III) on the surface of FSSR, followed by chemical adsorption of Cr(III). A comparison with biosorbents reported recently showed that FSSR exhibited competitive adsorption performance among the chemically modified biosorbents; although its maximum adsorption capacity was lower than that of some carbonized biosorbent, FSSR offers advantages in terms of cost-effectiveness and environmental sustainability, providing a feasible strategy for the valorization of agricultural residues and a sustainable alternative for the treatment of Cr(VI)-contaminated wastewater.
Graphical Abstract
References
- 1.
Wang, H.; Xu, J.; Liu, X.; et al. Preparation of straw activated carbon and its application in wastewater treatment: A review. J. Clean. Prod. 2021, 283, 124671.
- 2.
Chen, J.; Cai, Y.; Wang, Z.; et al. Construction of a synthetic microbial community for enzymatic pretreatment of wheat straw for biogas production via anaerobic digestion. Environ. Sci. Technol. 2024, 58, 9446–9455.
- 3.
Tao, W.; Yang, X.; Li, Y.; et al. Components and persistent free radicals in the volatiles during pyrolysis of lignocellulose biomass. Environ. Sci. Technol. 2020, 54, 13274–13281.
- 4.
Zhuo, H.; Dong, X.; Liu, Q.; et al. Bamboo-inspired ultra-strong nanofiber-reinforced composite hydrogels. Nat. Commun. 2025, 16, 980.
- 5.
Hou, D.; Jia, X.; Wang, L.; et al. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321.
- 6.
Zhao, K.; Zhang, W.; Liang, Z.; et al. Facilitating new chromium reducing microbes to enhance hexavalent chromium reduction by in situ sonoporation—Mediated gene transfer in soils. Environ. Sci. Technol. 2023, 57, 15123–15133.
- 7.
Thabede, P.M.; Shooto, N.D. Application of black cumin (Nigella sativa L.) seeds for the removal of metal ions and methylene blue from aqueous solutions. Cogent Eng. 2022, 9, 2013419.
- 8.
Vinayagam, R.; Dave, N.; Varadavenkatesan, T.; et al. Artificial neural network and statistical modelling of biosorptive removal of hexavalent chromium using macroalgal spent biomass. Chemosphere 2022, 296, 133965.
- 9.
Cho, S.; Jung, S.; Park, J.; et al. Strategic use of crop residue biochars for removal of hazardous compounds in wastewater. Bioresour. Technol. 2023, 387, 129658.
- 10.
Guerin, T.; Ghinet, A.; Hossart, M.; et al. Wheat and ryegrass biomass ashes as effective sorbents for metallic and organic pollutants from contaminated water in lab-engineered cartridge filtration system. Bioresour. Technol. 2020, 318, 124044.
- 11.
Han, F.; An, S.; Liu, L.; et al. Sulfoaluminate cement-modified straw biochar as a soil amendment to inhibit Pb-Cd mobility in the soil-remaine lettuce system. Chemosphere 2023, 332, 138891.
- 12.
Qu, J.; Wang, Y.; Tian, X.; et al. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292.
- 13.
Tian, S.; Shu, X.; Jiang, X.; et al. Mineralization and stabilization of toxic Pb2+ ions using CMC loaded S/P co-doped biochar composite. Sep. Purif. Technol. 2025, 354, 129161.
- 14.
Wu, Y.; Ming, J.; Zhou, W.; et al. Efficiency and mechanism in preparation and heavy metal cation/anion adsorption of amphoteric adsorbents modified from various plant straws. Sci. Total Environ. 2023, 884, 163887.
- 15.
Gkika, D.A.; Tolkou, A.K.; Katsoyiannis, I.A.; et al. The adsorption-desorption-regeneration pathway to a circular economy: The role of waste-derived adsorbents on chromium removal. Sep. Purif. Technol. 2025, 368, 132996.
- 16.
Rzig, B.; Guesmi, F.; Sillanpaa, M.; et al. Biosorption potential of olive leaves as a novel low-cost adsorbent for the removal of hexavalent chromium from wastewater. Biomass Convers. Biorefinery 2024, 14, 12961–12979.
- 17.
Zhen, H.G.; Hu, C.; Yang, L.; et al. Hydrochar produced from mixed feedstocks as efficient adsorbent for selenium and chromium removal from acidic wastewater. Desalination 2025, 593, 118152.
- 18.
Paglizccia, B.; Carretti, E.; Severi, M.; et al. Heavy metal biosorption by Extracellular Polymeric Substances (EPS) recovered from anammox granular sludge. J. Hazard. Mater. 2022, 424, 126661.
- 19.
Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; et al. Microalgal biosorption of heavy metals: A comprehensive bibliometric review. J. Hazard. Mater. 2021, 402, 123431.
- 20.
Wang, J.L.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226.
- 21.
Wang, P.; Yue, F.; Shao, C.; et al. Bio-sorption capacity of cadmium and zinc by Pseudomonas monteilii with heavy-metal resistance isolated from the compost of pig manure. Bioresour. Technol. 2024, 399, 130589.
- 22.
Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19.
- 23.
Ifthikar, J.; Zhao, M.; Shahzad, A.; et al. Recyclable process modeling study of hexavalent chromium elimination by thiol-based electron donor: Implications for practical applicability. J. Environ. Chem. Eng. 2021, 9, 105645.
- 24.
Kim, K.; Chung, H.Y.; Kim, B.; et al. Freezing-induced simultaneous reduction of chromate and production of molecular iodine: Mechanism, Kinetics, and practical Implications. Environ. Sci. Technol. 2020, 54, 16204–16211.
- 25.
Krishnani, K.K.; Meng, X.; Christodoulatos, C.; et al. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234.
- 26.
Liang, J.; Zhen, P.; Liu, L.; et al. Functional group-specific reduction of Cr(VI) by low molecular weight organic acids in frozen solution: Kinetics, mechanism and DFT calculation. Water Res. 2024, 265, 122221.
- 27.
Wang, W.; Fang, X.; Fu, Q.; et al. Iron(II/III) alters the relative roles of the microbial byproduct and humic acid during chromium(VI) reduction and fixation by soil-dissolved organic matter. Environ. Sci. Technol. 2025, 59, 2778–2790.
- 28.
Li, S.Z.; Chan-Halbrendt, C. Ethanol production in (the) People’s Republic of China: Potential and technologies. Appl. Energy 2009, 86, S162–S169.
- 29.
Wu, J.; Dong, J.; Wang, J. Adsorptive removal of Cu(II) from aqueous solution by fermented sweet sorghum residues as a novel biosorbent. J. Mol. Liq. 2022, 367, 120362.
- 30.
Wang, J.L.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156.
- 31.
Wang, J.L.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732.
- 32.
Wang, J.L.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279.
- 33.
Wang, J.L.; Guo, X. Adsorption kinetics and isotherm models of heavy metals by various adsorbents: An overview. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1837–1865.
- 34.
Richard, F.C.; Bourg, A.C.M. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816.
- 35.
Foday, E.H.; Bo, B.; Xu, X. Removal of toxic heavy metals from contaminated aqueous solutions using seaweeds: A review. Sustainability 2021, 13, 12311.
- 36.
Verma, R.; Maji, P.K.; Sarkar, S. Comprehensive investigation of the mechanism for Cr(VI) removal from contaminated water using coconut husk as a biosorbent. J. Clean. Prod. 2021, 314, 128117.

This work is licensed under a Creative Commons Attribution 4.0 International License.


