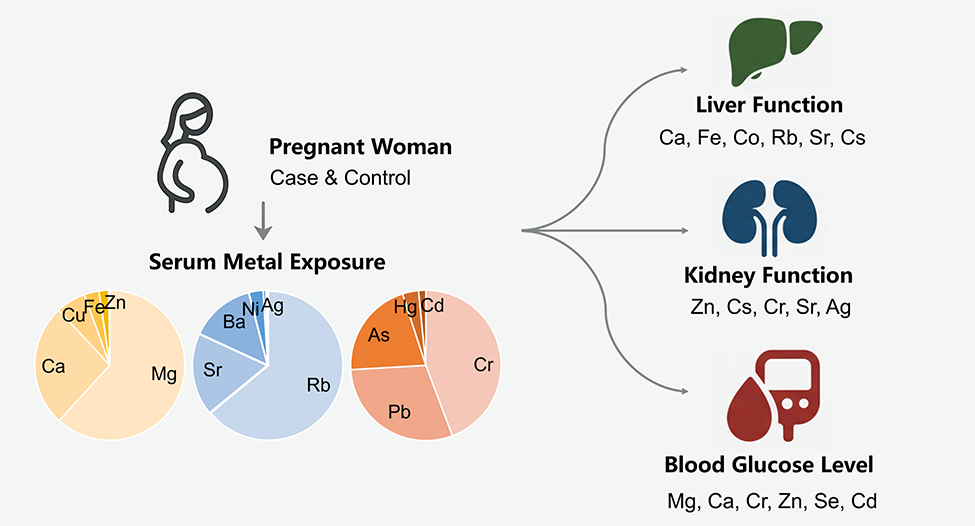
- Mg and Ca account for 88.28% of the total content of eight essential metals
- Elevated prenatal exposure to Hg and Ag linked to elevated health risks
- Ca, Fe, Co, Rb, Sr, and Cs were associated with liver function parameters
- Mg, Ca, Cr, Zn, Se, and Cd were associated with blood glucose regulation
- Cr, Zn, Sr, and Ag concentrations were related to uric acid levels
- Open Access
- Article
Metal Exposure in Chinese Pregnant Women from Dalian and Association with Clinical Indicators
- Yubing Dai 1, 2, †,
- Yuan Gao 1, †,
- Dongying Zheng 3,
- Jing Jin 1,
- Ningbo Geng 1, *,
- Haijun Zhang 1,
- Jiping Chen 1
Author Information
Received: 03 Jul 2025 | Revised: 06 Sep 2025 | Accepted: 24 Sep 2025 | Published: 14 Oct 2025
Highlights
Abstract
Maternal serum metal levels can significantly affect pregnancy outcomes and fetal health. This study investigated the associations between serum heavy metal concentrations and hematological parameters in 209 pregnant women in Dalian. Mg and Ca were the predominant metals, with concentrations of 18,493 μg/L and 8060 μg/L, respectively, comprising 88.28% of the total concentration of eight essential metals. Notably, toxic metals such as Cr and Pb show levels comparable to possibly essential trace metals like Co and Mn, highlighting the necessity of enhanced surveillance to mitigate health hazards. Analysis of serum metal concentrations and composition profiles in serum of pregnant woman between case and control groups using multiple linear regression, revealed that elevated prenatal exposure to Hg and Ag was associated with significantly increased health risks. Levels of Ca, Fe, Co, Rb, Sr, and Cs correlated with liver function markers, while Zn and Cs correlated with kidney function indicators. Cr, Zn, Sr, and Ag concentrations were associated with uric acid levels. After adjusting for multiple metals and potential confounders, statistical associations were found between specific metals (e.g., Mg, Ca, Cr, Zn, Se, Cd) and blood glucose levels, indicating a potential link to glucose metabolism. These findings may inform targeted interventions for gestational diabetes and liver function management in pregnant women. Further research is required to elucidate these relationships and their implications for pregnancy outcomes.
Graphical Abstract
Keywords
multiple metals | pregnancy health risks | serum biomarkers | gestational metabolic disruption
References
- 1.World Health Organization. WHO Human Health Risk Assessment Toolkit: Chemical Hazards; World Health Organization: Geneva, Switzerland, 2010.
- 2.Weyde, K.V.F.; Olsen, A.K.; Duale, N.; et al. Gestational blood levels of toxic metal and essential element mixtures and associations with global DNA methylation in pregnant women and their infants. Sci. Total Environ. 2021, 787, 147621.
- 3.Liu, D.; Shi, Q.; Liu, C.; et al. Effects of Endocrine-Disrupting Heavy Metals on Human Health. Toxics 2023, 11, 322.
- 4.Ismanto, A.; Hadibarata, T.; Kristanti, R.A.; et al. Endocrine disrupting chemicals (EDCs) in environmental matrices: Occurrence, fate, health impact, physio-chemical and bioremediation technology. Environ. Pollut. 2022, 302, 119061.
- 5.Ashrap, P.; Watkins, D.J.; Mukherjee, B.; et al. Predictors of urinary and blood Metal(loid) concentrations among pregnant women in Northern Puerto Rico. Environ. Res. 2020, 183, 109178.
- 6.Punshon, T.; Li, Z.; Marsit, C.J.; et al. Placental Metal Concentrations in Relation to Maternal and Infant Toenails in a U.S. Cohort. Environ. Sci. Technol. 2016, 50, 1587–1594.
- 7.Onat, T.; Demir Caltekin, M.; Turksoy, V.A.; et al. The Relationship Between Heavy Metal Exposure, Trace Element Level, and Monocyte to HDL Cholesterol Ratio with Gestational Diabetes Mellitus. Biol. Trace Elem. Res. 2020, 199, 1306–1315.
- 8.Duan, W.; Xu, C.; Liu, Q.; et al. Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: A population-based cohort study. Environ. Pollut. 2020, 263, 114630.
- 9.Bank-Nielsen, P.; Long, M.; Bonefeld-Jørgensen, E. Pregnant Inuit Women’s Exposure to Metals and Association with Fetal Growth Outcomes: ACCEPT 2010–2015. Int. J. Environ. Res. Public Health 2019, 16, 1171.
- 10.Fagher, U.; Laudanski, T.; Schütz, A.; et al. The relationship between cadmium and lead burdens and preterm labor. Int. J. Gynecol. Obstet. 1993, 40, 109–114.
- 11.Centers for Disease Control and Prevention. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010. Available online: https://stacks.cdc.gov/view/cdc/147837/cdc_147837_DS1.pdf (accessed on 12 January 2021).
- 12.Nordberg, G.; Sandström, B.; Becking, G.; et al. Essentiality and toxicity of trace elements: Principles and methods for assessment of risk from human exposure to essential trace elements. J. Trace Elem. Exp. Med. Off. Publ. Int. Soc. Trace Elem. Res. Hum. 2001, 14, 261–273.
- 13.Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; et al. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289.
- 14.Shah, D.; Sachdev, H.P.S. Effect of gestational zinc deficiency on pregnancy outcomes: Summary of observation studies and zinc supplementation trials. Br. J. Nutr. 2007, 85, S101–S108.
- 15.Abu-Saad, K.; Fraser, D. Maternal Nutrition and Birth Outcomes. Epidemiol. Rev. 2010, 32, 5–25.
- 16.Lin, C.-M.; Doyle, P.; Wang, D.; et al. The role of essential metals in the placental transfer of lead from mother to child. Reprod. Toxicol. 2010, 29, 443–446.
- 17.Cheong, J.N.; Wlodek, M.E.; Moritz, K.M.; et al. Programming of maternal and offspring disease: Impact of growth restriction, fetal sex and transmission across generations. J. Physiol. 2016, 594, 4727–4740.
- 18.Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331.
- 19.Wang, C.; Yu, A.; An, Y. Investigation and analysis of trace element detection in 1000 pregnant women in Guiyang. Res. Trace Elem. Health 2013, 30, 16–17. (In Chinese)
- 20.Liu, S.; Xie, K.; Jiang, H.; et al. Study on the relationship between serum trace elements in pregnant women and intrauterine growth retardation of fetus. J. Yan’an Univ. 2009, 7, 83–84. (In Chinese)
- 21.Xu, R.; Meng, X.; Pang, Y.; et al. Associations of maternal exposure to 41 metals/metalloids during early pregnancy with the risk of spontaneous preterm birth: Does oxidative stress or DNA methylation play a crucial role? Environ. Int. 2022, 158, 106966.
- 22.Tian, T.; Yin, S.; Jin, L.; et al. Single and mixed effects of metallic elements in maternal serum during pregnancy on risk for fetal neural tube defects: A Bayesian kernel regression approach. Environ. Pollut. 2021, 285, 117203.
- 23.Xu, C.; Xu, J.; Zhang, X.; et al. Serum nickel is associated with craniosynostosis risk: Evidence from humans and mice. Environ. Int. 2021, 146, 106289.
- 24.Hornung, R.W.; Reed, L.D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51.
- 25.Restrepo, B.I.; Camerlin, A.J.; Rahbar, M.H.; et al. Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis cases. Bull. World Health Organ. 2011, 89, 352–359.
- 26.Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; et al. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129.
- 27.Ying, Y.; Yu, C.; Yu, S.; et al. Trace element abundance analysis in serum of pregnant women. Shanghai J. Prev. Med. 2006, 12, 605–606. (In Chinese)
- 28.Wastney, M.E.; Aamodt, R.L.; Rumble, W.F.; et al. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1986, 251, R398–R408.
- 29.Jia, X.; Wei, J.; Du, H.. Effects of trace element levels in pregnant women on retinopathy of prematurity. J. Clin. Exp. Med. 2015, 14, 67–69. (In Chinese)
- 30.Zhou, Z.; Chen, G.; Li, P.; et al. Prospective association of metal levels with gestational diabetes mellitus and glucose: A retrospective cohort study from South China. Ecotoxicol. Environ. Saf. 2021, 210, 111854.
- 31.Ma, J.; Zhang, H.; Zheng, T.; et al. Exposure to metal mixtures and hypertensive disorders of pregnancy: A nested case-control study in China. Environ. Pollut. 2022, 306, 119439.
- 32.Liang, C.M.; Wu, X.Y.; Huang, K.; et al. Trace element profiles in pregnant women’s sera and umbilical cord sera and influencing factors: Repeated measurements. Chemosphere 2019, 218, 869–878.
- 33.Zhao, L.; Xu, H.; Yan, C.; et al. Study on the relationship between heavy metal elements such as lead and mercury and the occurrence of nervous system malformations. Chin. J. Eugen. Genet. 2008, 5, 94–96+107. (In Chinese)
- 34.Forsyth, J.E.; Weaver, K.L.; Maher, K.; et al. Sources of Blood Lead Exposure in Rural Bangladesh. Environ. Sci. Technol. 2019, 53, 11429–11436.
- 35.Luo, X.; Ding, J.; Xu, B.; et al. Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Sci. Total Environ. 2012, 424, 88–96.
- 36.Bradham, K.D.; Nelson, C.M.; Kelly, J.; et al. Relationship Between Total and Bioaccessible Lead on Children’s Blood Lead Levels in Urban Residential Philadelphia Soils. Environ. Sci. Technol. 2017, 51, 10005–10011.
- 37.Datko-Williams, L.; Wilkie, A.; Richmond-Bryant, J. Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci. Total Environ. 2014, 468–469, 854–863.
- 38.Liang, M. Analysis of blood magnesium concentration in patients with gestational diabetes mellitus. Chin. J. Eugen. Genet. 2010, 18, 69–83. (In Chinese)
- 39.Villar, J.; Belizán, J.M. Same nutrient, different hypotheses: Disparities in trials of calcium supplementation during pregnancy. Am. J. Clin. Nutr. 2000, 71, 1375S–1379S.
- 40.Loguercio, C.; De Girolamo, V.; Federico, A.A.; et al. Trace Elements and Chronic Liver Diseases. J. Trace Elem. Med. Biol. 1997, 11, 158–161.
- 41.Zhao, M.; Ge, X.; Xu, J.; et al. Association between urine metals and liver function biomarkers in Northeast China: A cross-sectional study. Ecotoxicol. Environ. Saf. 2022, 231, 113163.
- 42.Nangliya, V.; Sharma, A.; Yadav, D.; et al. Study of Trace Elements in Liver Cirrhosis Patients and Their Role in Prognosis of Disease. Biol. Trace Elem. Res. 2015, 165, 35–40.
- 43.Kaviani, S.; Izadyar, M.; Khavani, M.; et al. A combined molecular dynamics and quantum mechanics study on the interaction of Fe3+ and human serum albumin relevant to iron overload disease. J. Mol. Liq. 2020, 317, 113933.
- 44.Payne, R.B.; Little, A.J.; Williams, R.B.; et al. Interpretation of serum calcium in patients with abnormal serum proteins. Br. Med. J. 1973, 4, 643–646.
- 45.Wang, L.; Cao, C. Determination of serum calcium and albumin in pregnant women with pregnancy-induced hypertension and the study of their correlation. Contemp. Med. 2011, 17, 71–72.
- 46.Amirtharaj, G.J.; Natarajan, S.K.; Mukhopadhya, A.; et al. Fatty acids influence binding of cobalt to serum albumin in patients with fatty liver. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2008, 1782, 349–354.
- 47.Li, A.; Zhou, Q.; Mei, Y.; et al. The effect of urinary essential and non-essential elements on serum albumin: Evidence from a community-based study of the elderly in Beijing. Front. Nutr. 2022, 9, 946245.
- 48.Tsai, H.J.; Wu, P.Y.; Huang, J.C.; et al. Environmental Pollution and Chronic Kidney Disease. Int. J. Med. Sci. 2021, 18, 1121–1129.
- 49.Navarro-Alarcon, M.; Reyes-Pérez, A.; Lopez-Garcia, H.; et al. Longitudinal study of serum zinc and copper levels in hemodialysis patients and their relation to biochemical markers. Biol. Trace Elem. Res. 2006, 113, 209–222.
- 50.Prasad, A.S.; Bao, B.; Beck, F.W.J.; et al. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004, 37, 1182–1190.
- 51.Feig, D.I. Uric acid: A novel mediator and marker of risk in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2009, 18, 526–530.
- 52.Liu, T.; Zhang, M.; Rahman, M.L.; et al. Exposure to heavy metals and trace minerals in first trimester and maternal blood pressure change over gestation. Environ. Int. 2021, 153, 106508
- 53.Itoh, K.; Kawasaki, T.; Nakamura, M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br. J. Nutr. 2007, 78, 737–750.
- 54.Kesteloot, H.; Geboers, J. Calcium and blood pressure. Lancet 1982, 319, 813–815.
- 55.Allender, P.S.; Cutler, J.A.; Follmann, D.; et al. Dietary calcium and blood pressure: A meta-analysis of randomized clinical trials. Ann. Intern. Med. 1996, 124, 825–831.
- 56.Darroudi, S.; Saberi-Karimian, M.; Tayefi, M.; et al. Association Between Hypertension in Healthy Participants and Zinc and Copper Status: A Population-Based Study. Biol. Trace Elem. Res. 2018, 190, 38–44.
- 57.Li, Z.; Wang, W.; Liu, H.; et al. The association of serum zinc and copper with hypertension: A meta-analysis. J. Trace Elem. Med. Biol. 2019, 53, 41–48.
- 58.Mousavi, S.M.; Mofrad, M.D.; Nascimento, I.J.B.; et al. The effect of zinc supplementation on blood pressure: A systematic review and dose–response meta-analysis of randomized-controlled trials. Eur. J. Nutr. 2020, 59, 1815–1827.
- 59.Gao, C.; Sun, X.; Lu, L.; et al. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J. Diabetes Investig. 2018, 10, 154–162.
- 60.Hans, C.P.; Sialy, R.; Bansal, D.D. Magnesium deficiency and diabetes mellitus. Curr. Sci. 2002, 83, 1456–1463.
- 61.Kareem, I.; Jaweed, S.A.; Bardapurkar, J.S.; et al. Study of magnesium, glycosylated hemoglobin and lipid profile in diabetic retinopathy. Indian J. Clin. Biochem. 2004, 19, 124–127.
- 62.Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21.
- 63.Resnick, L.M. Hypertension and abnormal glucose homeostasis: Possible role of divalent ion metabolism. Am. J. Med. 1989, 87, S17–S22.
- 64.Chausmer, A.B. Zinc, Insulin and Diabetes. J. Am. Coll. Nutr. 1998, 17, 109–115.
- 65.Li, Z.; Xu, Y.; Huang, Z.; et al. Association between exposure to arsenic, nickel, cadmium, selenium, and zinc and fasting blood glucose levels. Environ. Pollut. 2019, 255, 113325.
- 66.Lin, J.; Shen, T. Association of dietary and serum selenium concentrations with glucose level and risk of diabetes mellitus: A cross sectional study of national health and nutrition examination survey, 1999–2006. J. Trace Elem. Med. Biol. 2021, 63, 126660.
- 67.Edwards, J.R.; Prozialeck, W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009, 238, 289–293.
- 68.Guimaraes, M.M.; Martins, A.C.; Silva, M.S. Chromium nicotinate has no effect on insulin sensitivity, glycemic control, and lipid profile in subjects with type 2 diabetes. J. Am. Coll. Nutr. 2013, 32, 243–250.

This work is licensed under a Creative Commons Attribution 4.0 International License.


