- Preparation methods of the catalysts for halogenated volatile organic compounds elimination are summarized.
- Catalytic elimination of halogenated volatile organic compounds is reviewed.
- Catalytic removal mechanisms of halogenated volatile organic compounds are discussed.
- Prospects and challenges of catalytic halogenated volatile organic compounds elimination are proposed.
- Open Access
- Review
Catalytic Elimination of Typical Halogenated Volatile Organic Compounds (HVOCs): A Critical Review
- Qinpei Sun 1,
- Xiaohui Yu 2,
- Keying Shang 1,
- Linke Wu 1,
- Peiqi Chu 1,
- Yuxi Liu 1,3,
- Jiguang Deng 1,3,
- Zhiquan Hou 1,3,
- Hongxing Dai 1,3,*
Author Information
Received: 14 Oct 2025 | Revised: 06 Nov 2025 | Accepted: 14 Nov 2025 | Published: 20 Nov 2025
Highlights
Abstract
Halogenated volatile organic compounds (HVOCs) represent a class of highly toxic, stable, and recalcitrant organic compounds among the volatile organic compounds (VOCs), posing big threats to air quality and human health. Catalytic elimination is a promising approach for HVOCs purification. However, catalytic elimination of HVOCs generates highly reactive and corrosive HX and X2 (X = F, Cl, and Br), giving rise to the poisoning and deactivation of catalysts. Moreover, the formation of numerous halogen-containing byproducts can induce secondary pollution issues. Therefore, the development of catalysts with high stability, good catalytic performance, and low selectivity towards toxic byproducts has been the primary research focus in the past years. Modifying geometric and electronic structures of the catalysts and constructing bifunctional catalysts with synergistic acidity and redox properties have demonstrated good catalytic performance and resistance towards poisoning. Depending on the category of HVOCs, different catalytic reaction systems are often required. The present review article summarizes recent progresses in catalysts preparation and their catalytic HVOCs elimination performance. By categorizing HVOCs removal into several specific reaction systems, we discuss the deep elimination efficiency of HVOCs, the selectivity of inorganic halogen species, and the migration pathways and removal mechanisms of halogens on the catalysts surface, thereby establishing the structure–performance relationships. Furthermore, we outline the prospects and challenges of catalytic HVOCs elimination technologies in the future work and practical applications. It is expected that the findings summarized in this review will inspire more researchers to develop catalytic systems capable of effectively eliminating HVOCs.
Graphical Abstract
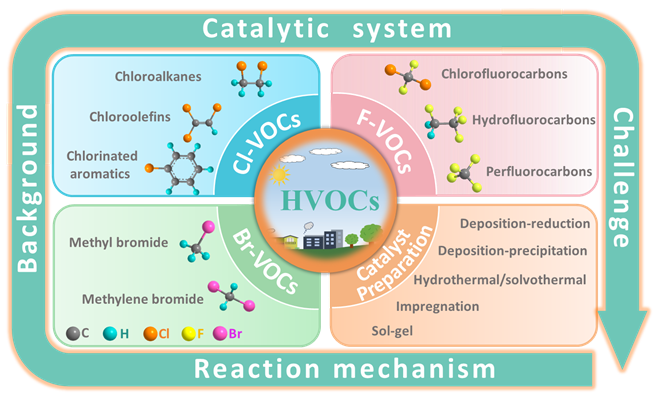
Keywords
halogenated volatile organic compound | catalytic elimination | catalyst preparation method | catalytic reaction mechanism | catalyst deactivation
References
- 1.He, C.; Cheng, J; Zhang, X.; et al. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568.
- 2.Jia, H.; Xing, Y.; Zhang, L.; et al. Progress of catalytic oxidation of typical chlorined volatile organic compounds (CVOCs): A review. Sci. Total Environ. 2023, 865, 161063.
- 3.Han, W.; Kennedy, E.; Mackie, J.; et al. Conversion of a CFCs, HFCs and HCFCs waste mixture via reaction with methane. J. Hazard. Mater. 2010, 184, 696–703.
- 4.Yu, H.; Kennedy, E.; Adesinab, A.; et al. A review of CFC and halon treatment technologies—The nature and role of catalysts. Catal. Surv. Asia 2006, 10, 40–54.
- 5.Chen, Y.; Qu, W.; Luo, T.; et al. Promoting C–F bond activation via proton donor for CF4 decomposition. Proc. Natl. Acad. Sci. USA 2024, 120, e2312480120.
- 6.Meng, X.; Dong, B.; Zhao, L.; et al. Synergistic regulation of charge state and electron-donating ability via heterojunctions design for fixation of electronegative greenhouse F-gases. Appl. Catal. B 2024, 364, 123709.
- 7.Paunović, V.; Pérez-Ramírez, J. Catalytic halogenation of methane: A dream reaction with practical scope? Catal. Sci. Technol. 2019, 9, 4515–4530.
- 8.Paunović, V.; Hemberger, P.; Bodi, A.; et al. Evidence of radical chemistry in catalytic methane oxybromination. Nat. Catal. 2018, 1, 363–370.
- 9.He, J.; Xu, T.; Wang, Z.; et al. Transformation of methane to propylene: A two-step reaction route catalyzed by modified CeO2 nanocrystals and zeolites. Angew. Chem. Int. Ed. 2012, 51, 2438–2442.
- 10.Gao, G.; Wei, L.; Liu, Z.; et al. Electron donation from boron suboxides via strong p–d orbital hybridization boosts molecular O2 activation on Ru/TiO2 for low-temperature dibromomethane oxidation. Environ. Sci. Technol. 2023, 57, 17566–17576.
- 11.Molina, M.J.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nature 1974, 249, 810–812.
- 12.Lin, H.; Liu, Y.; Deng, J.; et al. The advancement of supported bimetallic catalysts for the elimination of chlorinated volatile organic compounds. Catalysts 2024, 14, 531.
- 13.Sheraz, M.; Anus, A.; Le, V.C.T.; et al. A comprehensive review of contemporary strategies and approaches for the treatment of HFC-134a. Greenh. Gases 2021, 11, 1118–1133.
- 14.Takita, Y.; Tanabe, T.; Ito, M.; Decomposition of CH2FCF3 (134a) over metal phosphate catalysts. Ind. Eng. Chem. Res. 2002, 41, 2585–2590.
- 15.Ma, Z.; Hua, W.; Tang, Y; et al. Catalytic hydrolysis of CFC-12 over solid acid Ti(SO4)2. Chin. Chem. Lett. 2000, 11, 311–314.
- 16.Fu, X.; Zeltner, W.A.; Yang, Q; et al. Catalytic hydrolysis of dichlorodifluoromethane (CFC-12) on sol-gel-derived titania unmodified and modified with H2SO4. J. Catal. 1997, 168, 482–490.
- 17.Ma, Z.; Hua, W.; Tang, Y; et al. Catalytic decomposition of CFC-12 on solid acids SO42−/MxOy (M = Zr, Ti, Sn, Fe, Al). Chin. J. Chem. 2000, 18, 241–245.
- 18.Ma, Z.; Hua, W.; Tang, Y.; et al. Catalytic decomposition of CFC-12 over solid acids WO3/MxOy (M = Ti, Sn, Fe). J. Mol. Catal. A 2000, 159, 335–345.
- 19.Ding, S.; Wu, S.; Wang, P.; et al. Structure-selectivity relevance of multiple-component catalysts for CVOCs’ complete oxidation: State-of-the-art and perspectives. Sep. Purif. Technol. 2025, 354, 128964.
- 20.Yu, X.; Dai, L.; Deng, J.; et al. Catalytic performance and intermediates identification of trichloroethylene deep oxidation over Ru/3DOM SnO2 catalysts. J. Catal. 2021, 400, 310–324.
- 21.Yu, X.; Dai, L.; Peng, Y.; et al. High selectivity to HCl for the catalytic removal of 1,2-dichloroethane over RuP/3DOM WOx: Insights into the effects of P-doping and H2O introduction. Environ. Sci. Technol. 2021, 55, 14906–14916.
- 22.Wu, L.; Liu, Y.; Yu, X.; et al. Constructing bridge hydroxyl groups on the Ru/MOx/HZSM-5 (M = W, Mo) catalysts to promote the hydrolysis oxidation of multicomponent VOCs. Environ. Sci. Technol. 2024, 59, 945–955.
- 23.Wu, L.; Deng, J.; Liu, Y.; et al. Enhanced removal efficiency of multicomponent VOCs over the Sn-doped silicalite-1-supported Ru Single-atom catalysts by constructing tightly coupled redox and acidic sites. Appl. Catal. B 2024, 351, 123910.
- 24.Yu, X.; Deng, J.; Liu, Y.; et al. Enhanced water resistance and catalytic performance of Ru/TiO2 by regulating Brønsted acid and oxygen vacancy for the oxidative removal of 1,2-dichloroethane and toluene. Environ. Sci. Technol. 2022, 56, 11739–11749.
- 25.Liu, X.; Zeng, J.; Wang, J.; et al. Catalytic oxidation of methyl bromide using ruthenium-based catalysts. Catal. Sci. Technol. 2016, 6, 4337–4345.
- 26.Lv, L.; Wang, S.; Ding, Y.; et al. Reaction mechanism dominated by the Hard-Soft Acid-Base theory for the oxidation of CH2Cl2 and CH3Br over a titanium oxide-supported Ru catalyst. Ind. Eng. Chem. Res. 2020, 59, 7383–7388.
- 27.Lv, L.; Wang, S.; Ding, Y.; et al. Mechanistic insights into the contribution of Lewis acidity to brominated VOCs combustion over titanium oxide supported Ru catalyst. Chemosphere 2021, 263, 128112.
- 28.Tian, R.; Lu, J.; Xu, Z.; et al. Unraveling the synergistic reaction and the deactivation mechanism for the catalytic degradation of double components of sulfur-containing VOCs over ZSM-5-based materials. Environ. Sci. Technol. 2023, 57, 1443–1455.
- 29.Wang, X.; Li, Z.; Gao, R.; et al. Photothermal catalytic removal of 1,2-DCE with high HCl selectivity over the Brønsted acid-enriched sulfur-doped MOFs. Environ. Sci. Technol. 2024, 58, 17190–17200.
- 30.Zhang, C.; Gao, F.; Luo, N.; et al. Recent advances of chlorobenzene catalytic oxidation: influencing factors, roles of active sites and optimization. Sep. Purif. Technol. 2025, 376, 133962.
- 31.Feng, Y.; Jiang, Y.; Hua, M.; et al. Cooking oil fumes: A comprehensive review of emission characteristics and catalytic oxidation strategies. ACS EST Eng. 2025, 5, 303–324.
- 32.Li, Z.; Gao, R.; Hou, Z.; et al. Tandem supported Pt and ZSM-5 catalyst with separated catalytic functions for promoting multicomponent VOCs oxidation. Appl. Catal. B 2023, 339, 123131.
- 33.Maupin, I.; Pinard, L.; Mijoin, J.; et al. Bifunctional mechanism of dichloromethane oxidation over Pt/Al2O3: CH2Cl2 disproportionation over alumina and oxidation over platinum. J. Catal. 2012, 291, 104–109.
- 34.Bahareh, A.T.; Eskandari, S.; Khan, U.; et al. A review of preparation methods for supported metal catalysts. Adv. Catal. 2017, 61, 1–35.
- 35.Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718.
- 36.Zhang, Y.; Zhang, G.; Liu, J.; et al. Insight into the role of preparation method on the structure and size effect of Ni/MSS catalysts for dry reforming of methane. Fuel Process. Technol. 2023, 250, 107891.
- 37.Wang, J.; Liu, X.; Zeng, J.; et al. Catalytic oxidation of trichloroethylene over TiO2 supported ruthenium catalysts. Catal. Commun. 2016, 76,13–18.
- 38.Deraz, N.M. The comparative jurisprudence of catalysts preparation methods: II. Deposition-precipitation and adsorption methods. J. Ind. Environ. Chem. 2018, 2, 1–3.
- 39.Wang, T.; Liu, S.; Wang, L.; et al. High-performance Rh/CeO2 catalysts prepared by L-lysine-assisted deposition precipitation method for steam reforming of toluene. Fuel 2023, 341, 127736.
- 40.Simon, P.; Zanfoni, N.; Avril, L.; et al. Nanoporous platinum doped cerium oxides thin films grown on silicon substrates: Ionic platinum localization and stability. Adv. Mater. Interfaces 2017, 4, 1600821.
- 41.Hou, Z.; Lu, Y.; Liu, Y.; et al. A general dual-metal nanocrystal dissociation strategy to generate robust high-temperature-stable alumina-supported single-atom catalysts. J. Am. Chem. Soc. 2023, 145, 15869–15878.
- 42.Qiu, J.; Peng, Y.; Tang, M.; et al. Catalytic activity, selectivity, and stability of co-precipitation synthesized Mn-Ce mixed oxides for the oxidation of 1,2-dichlorobenzene. Environ. Sci. Pollut. Res. Int. 2021, 28, 65416–65427.
- 43.Wang, X.; Kang, Q.; Li, D. Catalytic combustion of chlorobenzene over MnOx–CeO2 mixed oxide catalysts. Appl. Catal. B 2009, 86, 166–175.
- 44.Feng, X.; Zheng, Y.; Lin, D.; et al. Novel synthetic route to Ce-Cu-W-O microspheres for efficient catalytic oxidation of vinyl chloride emissions. Chin. J. Catal. 2020, 41, 1864–1872.
- 45.Tian, M.; Jian, Y.; Ma, Y.; et al. Rational design of CrOx/LaSrMnCoO6 composite catalysts with superior chlorine tolerance and stability for 1,2-dichloroethane deep destruction. Appl. Catal. A 2019, 570, 62–72.
- 46.Liu, J.; Wang, Y.; Dai, Z.; et al. Recent advances in Zeolite-Based catalysts for volatile organic compounds decontamination by thermal catalytic oxidation. Sep. Purif. Technol. 2024, 330, 125339.
- 47.Wu, L.; Liu, Y.; Jia, Y.; et al. A novel strategy for enhancing resistance to chlorine, water, and sulfur oxide of the Pt/Co-ZSM-5 catalyst by synergistic coupling of acidity and redox sites for the oxidation of multicomponent VOCs. Appl. Catal. B 2025, 378, 125557.
- 48.Gołąbek, K.; Palomares, A.E.; Martínez-Triguero, J.; et al. Ce-modified zeolite BEA catalysts for the trichloroethylene oxidation. The role of the different and necessary active sites. Appl. Catal. B 2019, 259, 118022.
- 49.Sun, Q.; Yu, X.; Wu, L.; et al. Boosting catalytic and anti-fluorination performance of the Ru/vanadia–titania catalyst for the oxidative destruction of Freon by sulfuric acid modification. Environ. Sci. Technol. 2024, 58, 12719–12730.
- 50.Karmakar, S.; Greene, H.L. An investigation of CFC12 (CCl2F2) decomposition on TiO2 catalyst. J. Catal. 1995, 151, 394–406.
- 51.Li, Y.; Ren, Y.; Xiao, H.; et al. Recent advances of the effect of H2O on VOC oxidation over catalysts: Influencing factors, inhibition/promotion mechanisms, and water resistance strategies. Environ. Sci. Technol. 2025, 59, 1034–1059.
- 52.Zhang, H.; Luo, T.; Long, Y.; et al. Identification of the active site during CF4 hydrolytic decomposition over γ-Al2O3. Environ. Sci. Nano 2022, 9, 954–963.
- 53.Takita, Y.; Morita, C.; Ninomiya, M.; et al. Catalytic decomposition of CF4 over AlPO4-based catalyst. Chem. Lett. 1999, 417–418.
- 54.Zhang, H.; Liu, K.; Chen, Y.; et al. Efficient and stable CF4 decomposition over θ-Al2O3 with extraordinary resistance to HF. Environ. Sci. Nano 2023, 10, 3149–3155.
- 55.Luo, T.; Chen, Y.; Liu, K.; et al. Rational design of active sites in alumina-based catalysts to optimize antibonding-orbital occupancy for tetrafluoromethane decomposition. Environ. Sci. Nano 2023, 10, 3307–3316.
- 56.Xu, X.; Jeon, J.Y.; Choi, M.H.; et al. The modification and stability of γ-Al2O3 based catalysts for hydrolytic decomposition of CF4. J. Mol. Catal. A. 2007, 266, 131–138.
- 57.Jeon, H.; Oh, M.; Han, J.W.; et al. Understanding remarkable promotional effects of Zn on alumina in catalytic hydrolysis of perfluorocarbon. J. Catal. 2023, 426, 361–367.
- 58.Li, Z.; Tan, X.; Ren, G.; et al. Equivalence of difluorodichloromethane (CFC-12) hydrolysis catalyzed by solid acid (base) MoO3(MgO)/ZrO2. RSC Adv. 2020, 10, 33662–33674.
- 59.Takita, Y.; Wakamatsu, H.; Tokumaru, M.; et al. Decomposition of chlorofluorocarbons over metal phosphate catalysts III.: Reaction path of CCl2F2 decomposition over AlPO4. Appl. Catal. A 2000, 194, 55–61.
- 60.Ning, P.; Wang, X.; Bart, H.; et al. Catalytic decomposition of CFC-12 over solid superacid Mo2O3/ZrO2. J. Environ. Eng. 2011, 137, 897–902.
- 61.Han, T.U.; Yoo, B.S.; Kim, Y.M.; et al. Catalytic conversion of 1,1,1,2-tetrafluoroethane (HFC-134a). Korean J. Chem. Eng. 2018, 35, 1611–1619.
- 62.Swamidoss, C.M.A.; Sheraz, M.; Anus, A.; et al. Effect of Mg/Al2O3 and calcination temperature on the catalytic decomposition of HFC-134a. Catalysts 2019, 9, 270.
- 63.Kim, M.J.; Kim, Y.; Youn, J.R.; et al. Effects of sulfuric acid treatment on the performance of Ga-Al2O3 for the hydrolytic decomposition of 1,1,1,2-tetrafluoroethane (HFC-134a). Catalysts 2020, 10, 766.
- 64.El-Bahy, Z.; Ohnishi, R.; Ichikawa, M. Hydrolysis of CF4 over alumina-based binary metal oxide catalysts. Appl. Catal. B 2003, 40, 81–91.
- 65.Digne, M.; Sautet, P.; Raybaud, P.; et al. Hydroxyl groups on γ-alumina surfaces: A DFT study. J. Catal. 2002, 211, 1–5.
- 66.Nortier, P.; Fourre, P.; Mohammed Saad, A.B.; et al. Effects of crystallinity and morphology on the surface properties of alumina. Appl. Catal. 1990, 61, 141–160.
- 67.Murrayrust, P.; Stallings, W.; Monti, C.; et al. Intermolecular interactions of the C–F bond: The crystallographic environment of fluorinated carboxylic acids and related structures. J. Am. Chem. Soc. 1983, 105, 3206–3214.
- 68.Cormanich, R.A.; Rittner, R.; Freitas, M.P.; et al. The seeming lack of CF…HO intramolecular hydrogen bonds in linear aliphatic fluoroalcohols in solution. Phys. Chem. Chem. Phys. 2014, 16, 19212–19217.
- 69.Luo, T.; Zhang, H.; Chen, Y.; et al. Unveiling tetrafluoromethane decomposition over alumina catalysts. J. Am. Chem. Soc. 2024, 146, 35057–35063.
- 70.Wang, X.; Fu, J.; Zhang, H.; et al. Detoxification of carbonaceous species for efficient perfluorocarbon hydrolysis. Environ. Sci. Technol. 2025, 59, 3309–3315.
- 71.Takita, Y.; Moriyama, J.; Yoshinaga, Y.; et al. Adsorption of water vapor on the AlPO4-based catalysts and reaction mechanism for CFCs decomposition. Appl. Catal. A 2004, 271, 55–60.
- 72.Ng, C.F.; Shan, S.; Lai, S. Catalytic decomposition of CFC-12 on transition metal chloride promoted γ-alumina. Appl. Catal. B 1998, 16, 209–217.
- 73.Gong-Liang, L.; Hiroyasu, N.; Tatsumi, I.; et al. Catalytic dehydrouorination of CF3CH3(HFC143a) into CF2CH2(HFC1132a). Appl. Catal. B 1998, 16, 309–317.
- 74.Tu, C.; Zhang, H.; Wang, X.; et al. Phase-Engineered ZrO2 for tuning catalytic oxidation of dichloromethane over W/ZrO2: Zr-doped WOx clusters and the hydrolysis-oxidation mechanism. Environ. Sci. Technol. 2025, 58, 2838−2848.
- 75.Wang, W.; Zhu, Q.; Dai, Q.; et al. Fe doped CeO2 nanosheets for catalytic oxidation of 1,2-dichloroethane: Effect of preparation method. Chem. Eng. J. 2017, 307, 1037–1046.
- 76.Zhang, X.; Dai, L.; Liu, Y.; et al. Effect of support nature on catalytic activity of the bimetallic RuCo nanoparticles for the oxidative removal of 1,2-dichloroethane. Appl. Catal. B 2021, 285, 119804.
- 77.Tian, M.; Guo, X.; Dong, R.; et al. Insight into the boosted catalytic performance and chlorine resistance of nanosphere-like meso-macroporous CrOx/MnCo3Ox for 1,2-dichloroethane destruction. Appl. Catal. B 2019, 259, 118018.
- 78.Cao, S.; Wang, H.; Yu, F.; et al. Catalyst performance and mechanism of catalytic combustion of dichloromethane (CH2Cl2) over Ce doped TiO2. J. Colloid Interface Sci. 2016, 463, 233–241.
- 79.Dai, Q.; Zhang, Z.; Yan, J.; et al. Phosphate-functionalized CeO2 nanosheets for efficient catalytic oxidation of dichloromethane. Environ. Sci. Technol. 2018, 52, 13430–13437.
- 80.Zhang, X.; Liu, Y.; Deng, J.; et al. Alloying of gold with palladium: An effective strategy to improve catalytic stability and chlorine-tolerance of the 3DOM CeO2-supported catalysts in trichloroethylene combustion. Appl. Catal. B 2019, 257, 117879.
- 81.Gao, R.; Zhang, M.; Liu, Y.; et al. Engineering platinum catalysts via a site-isolation strategy with enhanced chlorine resistance for the elimination of multicomponent VOCs. Environ. Sci. Technol. 2022, 56, 9672−9682.
- 82.Zhang, X.; Liu, Y.; Deng, J.; et al. Three-dimensionally ordered macroporous Cr2O3−CeO2: High-performance catalysts for the oxidative removal of trichloroethylene. Catal. Today 2020, 339, 200–209.
- 83.Scirè, S.; Minicò, S.; Crisafulli, C. Pt catalysts supported on H-type zeolites for the catalytic combustion of chlorobenzene. Appl. Catal. B 2003, 45, 117–125.
- 84.He, C.; Yu, Y.; Shi, J.; et al. Mesostructured Cu–Mn–Ce–O composites with homogeneous bulk composition for chlorobenzene removal: Catalytic performance and microactivation course. Mater. Chem. Phys. 2015, 157, 87–100.
- 85.Gu, Y.; Cai, T.; Gao, X.; et al. Catalytic combustion of chlorinated aromatics over WOx/CeO2 catalysts at low temperature. Appl. Catal. B 2019, 248, 264–276.
- 86.Deng, Y.; Shang, Y.; Huang, T.; et al. Reversing the HCl/Cl2 selectivity for efficient catalytic elimination of dichloromethane by incorporation of Ti4+ into CeO2 lattice. Appl. Catal. B 2025, 373, 125338.
- 87.Van den Brink, R.W.; Mulder, P.; Louw, R.; et al. Catalytic oxidation of dichloromethane on γ-Al2O3: A combined flow and infrared spectroscopic study. J. Catal. 1998, 180, 153–160.
- 88.Yang, Y.; Liu, S.; Zhao, H.; et al. Promotional effect of doping Cu into cerium-titanium binary oxides catalyst for deep oxidation of gaseous dichloromethane. Chemosphere 2019, 214, 553–562.
- 89.Yin, L.; Lu, G.; Gong, X. A DFT+U study of the catalytic degradation of 1,2-dichloroethane over CeO2. Phys. Chem. Chem. Phys. 2018, 20, 5856–5864.
- 90.Fei, Z.; Cheng, C.; Chen, H.; et al. Construction of uniform nanodots CeO2 stabilized by porous silica matrix for 1,2-dichloroethane catalytic combustion. Chem. Eng. J. 2019, 370, 916–924.
- 91.Yang, P.; Xue, X.; Meng, Z.; et al. Enhanced catalytic activity and stability of Ce doping on Cr supported HZSM-5 catalysts for deep oxidation of chlorinated volatile organic compounds. Chem. Eng. J. 2013, 234, 203–210.
- 92.Lin, F.; Xiang, L.; Zhang, Z.; et al. Comprehensive review on catalytic degradation of Cl-VOCs under the practical application conditions. Crit. Rev. Environ. Sci. Technol. 2020, 52, 311–355.
- 93.Wang, L.; Wang, C.; Xie, H.; et al. Catalytic combustion of vinyl chloride over Sr doped LaMnO3. Catal. Today 2019, 327, 190–195.
- 94.Zhang, C.; Wang, C.; Zhan, W.; et al. Catalytic oxidation of vinyl chloride emission over LaMnO3 and LaB0.2Mn0.8O3 (B = Co, Ni, Fe) catalysts. Appl. Catal. B 2013, 129, 509–516.
- 95.Huang, H.; Dai, Q.; Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B 2014, 158–159, 96–105.
- 96.Van Den Brink, R.W.; Louw, R.; Mulder, P. Formation of polychlorinated benzenes during the catalytic combustion of chlorobenzene using a Pt/γ-Al203 catalyst. Appl. Catal. B 1998, 16, 219–226.
- 97.Sun, P.; Wang, W.; Dai, X.; et al. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1-xO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B 2016, 198, 389–397.
- 98.Gao, F.; Chen, D.; Luo, N.; et al. Catalytic performance and reaction mechanism of chlorobenzene oxidation over MnOx-CeO2 catalyst. Chem. J. Chin. Univ. 2023, 44, 20220690.
- 99.Long, G.; Chen, M.; Li, Y.; et al. One-pot synthesis of monolithic Mn-Ce-Zr ternary mixed oxides catalyst for the catalytic combustion of chlorobenzene. Chem. Eng. J. 2018, 360, 964–973.
- 100.Mei, J.; Xie, J.; Qu, Z.; et al. Ordered mesoporous spinel Co3O4 as a promising catalyst for the catalytic oxidation of dibromomethane. Mol. Catal. 2018, 461, 60–66.
- 101.Mei, J.; Ke, Y.; Yu, Z.; et al. Morphology-dependent properties of Co3O4/CeO2 catalysts for low temperature dibromomethane (CH2Br2) oxidation. Chem. Eng. J. 2017, 320, 124–134.
- 102.Mei, J.; Huang, W.; Qu, Z.; et al. Catalytic oxidation of dibromomethane over Ti-modified Co3O4 catalysts: Structure, activity and mechanism. J. Colloid Interface Sci. 2017, 505, 870–883.
- 103.Gao, G.; Hou, J.; Fan, Y.; et al. Stabilizing Ru-Based catalysts against bromine poisoning through Ru–O Covalency regulation for durable brominated volatile organic compound oxidation. Environ. Sci. Technol. 2025, 59, 15504–15514.
- 104.Mei, J.; Zhao, S.; Huang, W.; et al. Mn-Promoted Co3O4/TiO2 as an efficient catalyst for catalytic oxidation of dibromomethane (CH2Br2). J. Hazard. Mater. 2016, 318, 1–8.
- 105.Mei, J.; Xie, J.; Sun, Y.; et al. Design of Co3O4/CeO2–Co3O4 hierarchical binary oxides for the catalytic oxidation of dibromomethane. J. Ind. Eng. Chem. 2019, 73, 134–141.
- 106.Mei, J.; Qu, Z.; Zhao, S.; et al. Promoting effect of Mn and Ti on the structure and performance of Co3O4 catalysts for oxidation of dibromomethane. J. Ind. Eng. Chem. 2017, 57, 208–215.
- 107.Lv, L.; Wang, S.; Ding, Y.; et al. Deactivation mechanism and anti-deactivation modification of Ru/TiO2 catalysts for CH3Br oxidation. Chemosphere 2020, 257, 127249.
- 108.Chen, C.Y.; Pignatello, J.J. Catalytic oxidation for elimination of methyl bromide fumigation emissions using ceria-based catalysts. Appl. Catal. B 2013, 142–143, 785–794.

This work is licensed under a Creative Commons Attribution 4.0 International License.


