- Summarized advances in materials-based platforms for uranyl detection
- Highlighted the roles of advanced materials in uranyl removal
- Discussed AI integration and future perspectives in this field
- Open Access
- Review
Recent Advances in Material-Based Platforms for Rapid Uranyl Detection and Removal
- Wentao Zhang 1,
- Zhenli Sun 1,*,
- Suhua Wang 2,
- Xishi Tai 3,
- Xiangke Wang 1,*
Author Information
Received: 30 Aug 2025 | Revised: 14 Nov 2025 | Accepted: 19 Nov 2025 | Published: 24 Nov 2025
Highlights
Abstract
Nuclear energy is a key low-carbon source for global carbon neutrality, yet its rapid expansion has intensified uranium mining, fuel processing, and nuclear wastewater discharge, raising concerns about the environmental and health risks associated with uranyl (UO22+) contamination. Reliable monitoring and effective removal of uranium contamination are therefore essential for ensuring environmental safety and conducting accurate risk assessment. Advanced material-based sensors have emerged as promising solutions, owing to their high sensitivity, selectivity, portability, and rapid response. Recent advances feature platforms based on noble metals, quantum dots, metal–organic frameworks, covalent organic frameworks, and nanocomposites, which enable efficient uranyl removal and detection through optical, electrochemical, and multifunctional strategies. The integration of artificial intelligence for spectral interpretation and recognition is also discussed, highlighting its potential to overcome challenges in complex water matrices. This review further outlines perspectives on sustainable, field-deployable platforms for uranyl detection and removal, aiming to safeguard environmental health and support effective nuclear wastewater management.
Graphical Abstract
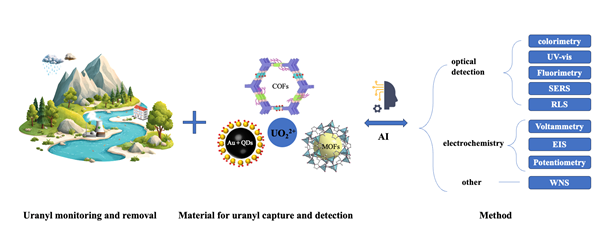
Keywords
uranyl | detection | removal | material | artificial intelligence
References
- 1.Sun, Z.L.; Chen, Z.S.; Wang, S.H.; et al. Nuclear energy: Where next? Innovation 2026, 7, 101093.
- 2.Tai, X.S.; Sun, Z.L. Extra-high extraction of uranium from seawater by covalent organic frameworks through structure geometry and functional active site modification. Sustain. Carbon. Mater. 2025, 1, e006.
- 3.Chen, T.; Liu, T.; Zhou, L.; et al. Ternary boron carbon nitrides hollow nanotubes with tunable p-n homojunction for photo-assisted uranium extraction: A combined batch, EXAFS and DFT calculations. Appl. Catal. B Environ. 2022, 318, 121815.
- 4.Chen, X.T.; He, L.F.; Wang, Y.; et al. Trace analysis of uranyl ion (UO22+) in aqueous solution by fluorescence turn-on detection via aggregation induced emission enhancement effect. Anal. Chim. Acta 2014, 847, 55–60.
- 5.Averseng, O.; Hagège, A.; Taran, F.; et al. Surface plasmon resonance for rapid screening of uranyl affine proteins. Anal. Chem. 2010, 82, 9797–9802.
- 6.Fukuda, S.; Ikeda, M.; Nakamura, M.; et al. Acute toxicity of subcutaneously administered depleted uranium and the effects of CBMIDA in the simulated wounds of rats. Health Phys. 2009, 96, 483–492.
- 7.Lourenço, J.; Pereira, R.; Gonçalves, F.; et al. Metal bioaccumulation, genotoxicity and gene expression in the European wood mouse (Apodemus sylvaticus) inhabiting an abandoned uranium mining area. Sci. Total Environ. 2013, 443, 673–680.
- 8.Selvakumar, R.; Ramadoss, G.; Menon, M.P.; et al. Challenges and complexities in remediation of uranium contaminated soils: A review. J. Environ. Radioact. 2018, 192, 592–603.
- 9.Yildiz, E.; Saçmaci, S.; Kartal, S.; et al. A new chelating reagent and application for coprecipitation of some metals in food samples by FAAS. Food Chem. 2016, 194, 143–148.
- 10.Balaram, V. Recent advances in the determination of elemental impurities in pharmaceuticals-status, challenges and moving frontiers. Trends Anal. Chem. 2016, 80, 83–95.
- 11.Santos, J.S.; Teixeira, L.S.G.; dos Santos, W.N.L.; et al. Uranium determination using atomic spectrometric techniques: An overview. Anal. Chim. Acta 2010, 674, 143–156.
- 12.Sanyal, K.; Khooha, A.; Das, G.; et al. Direct determination of oxidation states of uranium in mixed-valent uranium oxides using total reflection X-ray fluorescence X-ray absorption near-edge spectroscopy. Anal. Chem. 2017, 89, 871–876.
- 13.Bings, N.H.; Bogaerts, A.; Broekaert, J.A.C. Atomic spectroscopy: A review. Anal. Chem. 2010, 82, 4653–4681.
- 14.Hellé, G.; Mariet, C.; Cote, G. Liquid-liquid extraction of uranium(VI) with aliquat® 336 from HCl media in microfluidic devices: Combination of micro-unit operations and online ICP-MS determination. Talanta 2015, 139, 123–131.
- 15.Kalita, M.P.C.; Deka, K.; Das, J.; et al. X-ray diffraction line profile analysis of chemically synthesized lead sulphide nanocrystals. Mater. Lett. 2012, 87, 84–86.
- 16.Ajitha, B.; Reddy, Y.A.K.; Kim, M.J.; et al. Superior catalytic activity of synthesized triangular silver nanoplates with optimized sizes and shapes. Catal. Sci. Technol. 2016, 6, 8289–8299.
- 17.Nasrollahzadeh, M.; Zahraei, A.; Ehsani, A.; et al. Synthesis, characterization, antibacterial and catalytic activity of a nanopolymer supported copper(II) complex as a highly active and recyclable catalyst for the formamidation of arylboronic acids under aerobic conditions. RSC Adv. 2014, 4, 20351–20357.
- 18.Rasheed, T.; Bilal, M.; Nabeel, F.; et al. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485.
- 19.Ullah, N.; Mansha, M.; Khan, I.; et al. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trends Anal. Chem. 2018, 100, 155–166.
- 20.He, W.W.; Hua, D.B. Spectrographic sensors for uranyl detection in the environment. Talanta 2019, 201, 317–329.
- 21.Wu, X.M.; Huang, Q.X.; Mao, Y.; et al. Sensors for determination of uranium: A review. Trends Anal. Chem. 2019, 118, 89–111.
- 22.Chen, Z.; Zhang, Z.Y.; Qi, J.; et al. Colorimetric detection of heavy metal ions with various chromogenic materials: Strategies and applications. J. Hazard. Mater. 2023, 441, 129889.
- 23.Fan, Y.J.; Li, J.W.; Guo, Y.P.; et al. Digital image colorimetry on smartphone for chemical analysis: A review. Measurement 2021, 171, 108829.
- 24.Hou, D.B.; Zhang, J.; Chen, L.; et al. Water quality analysis by UV-Vis spectroscopy: A review of methodology and application. Spectrosc. Spectr. Anal. 2013, 33, 1839–1844.
- 25.Zhang, H.Y.; Ruan, Y.J.; Lin, L.; et al. A turn-off fluorescent biosensor for the rapid and sensitive detection of uranyl ion based on molybdenum disulfide nanosheets and specific DNAzyme. Spectrochim. Acta A 2015, 146, 1–6.
- 26.Wang, H.P.; Chen, P.; Dai, J.W.; et al. Recent advances of chemometric calibration methods in modern spectroscopy: Algorithms, strategy, and related issues. Trends Anal. Chem. 2022, 153, 116648.
- 27.Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.X.; et al. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577.
- 28.Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; et al. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 2022, 1, 87.
- 29.Zhou, B.; Shi, L.F.; Wang, Y.S.; et al. Resonance light scattering determination of uranyl based on labeled DNAzyme-gold nanoparticle system. Spectrochim. Acta A 2013, 110, 419–424.
- 30.Li, S.J.; Liao, L.F.; Wu, R.R.; et al. Resonance light scattering detection of fructose bisphosphates using uranyl-salophen complex-modified gold nanoparticles as optical probe. Anal. Bioanal. Chem. 2015, 407, 8911–8918.
- 31.Lu, Y.Y.; Liang, X.Q.; Niyungeko, C.; et al. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338.
- 32.Tang, X.; Han, H.; Li, L.; et al. Electrodes functionalized with advanced recognition materials for trace electrochemical sensing of uranyl ion. Microchem. J. 2024, 199, 109924.
- 33.Wang, S.S.; Zhang, J.B.; Gharbi, O.; et al. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 50.
- 34.Aragay, G.; Merkoçi, A. Nanomaterials application in electrochemical detection of heavy metals. Electrochim. Acta 2012, 84, 49–61.
- 35.Pacella, N.; DeRouin, A.; Pereles, B.; et al. Geometrical modification of magnetoelastic sensors to enhance sensitivity. Smart Mater. Struct. 2015, 24, 025018.
- 36.Zhang, H.; Lin, L.; Zeng, X.; et al. Magnetic beads-based DNAzyme recognition and AuNPs-based enzymatic catalysis amplification for visual detection of trace uranyl ion in aqueous environment. Biosens. Bioelectron. 2016, 78, 73–79.
- 37.Wu, X.M.; Mao, Y.; Wang, D.Y.; et al. Designing a colorimetric sensor containing nitrogen and oxygen atoms for uranyl ions identification: Chromatic mechanism, binding feature and on-site application. Sens. Actuators B 2020, 307, 127681.
- 38.Liang, Y.; He, Y. Arsenazo III-functionalized gold nanoparticles for photometric determination of uranyl ion. Microchim. Acta 2016, 183, 407–413.
- 39.Drogat, N.; Jauberty, L.; Chaleix, V.; et al. Sensing of the uranyl ion based on its complexation with bisphosphonate-capped gold nanoparticles. Mater. Lett. 2014, 122, 208–211.
- 40.Zhang, D.Y.; Chen, Z.; Omar, H.; et al. Colorimetric peroxidase mimetic assay for uranyl detection in sea water. ACS Appl. Mater. Interfaces 2015, 7, 4589–4594.
- 41.Saha, A.; Neogy, S.; Rao, D.R.M.; et al. Colorimetric and visual determination of ultratrace uranium concentrations based on the aggregation of amidoxime functionalized gold nanoparticles. Microchim. Acta 2019, 186, 183.
- 42.Zhang, L.S.; Huang, D.S.; Zhao, P.X.; et al. Colorimetric detection for uranyl ions in water using vinylphosphonic acid functionalized gold nanoparticles based on smartphone. Spectrochim. Acta A 2022, 269, 120748.
- 43.Xu, Y.L.; Wei, J.H.; Chen, X.W. Visible light-responsive sulfone-based covalent organic framework as metal-free nanoenzyme for visual colorimetric determination of uranium. Chemosensors 2022, 10, 248.
- 44.Xiao, S.J.; Huang, J.; Qiu, A.T.; et al. Advanced "turn-on" colorimetric uranium platform based on the enhanced nanozyme activity of a donor-acceptor structured covalent organic framework. Anal. Chim. Acta 2024, 1302, 342503.
- 45.Amini, A.; Khajeh, M.; Oveisi, A.R.; et al. A porous multifunctional and magnetic layered graphene oxide/3D mesoporous MOF nanocomposite for rapid adsorption of uranium(VI) from aqueous solutions. J. Ind. Eng. Chem. 2021, 93, 322–332.
- 46.Bai, F.; Yang, X.; Yang, C.; et al. Amidoxime covalent organic framework@Fe3O4 based magnetic solid-phase extraction for rapid and sensitive determination of trace uranium in seafood. J. Chromatogr. A 2025, 1740, 465564.
- 47.Zhang, J.J.; Cheng, F.F.; Li, J.J.; et al. Fluorescent nanoprobes for sensing and imaging of metal ions: Recent advances and future perspectives. Nano Today 2016, 11, 309–329.
- 48.Saha, A.; Debnath, T.; Neogy, S.; et al. Micellar extraction assisted fluorometric determination of ultratrace amount of uranium in aqueous samples by novel diglycolamide-capped quantum dot nanosensor. Sens. Actuators B 2017, 253, 592–602.
- 49.Liu, W.; Dai, X.; Bai, Z.L.; et al. Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal-organic framework equipped with abundant lewis basic sites: A combined batch, X-ray absorption spectroscopy, and first principles simulation investigation. Environ. Sci. Technol. 2017, 51, 3911–3921.
- 50.Liu, W.; Dai, X.; Wang, Y.L.; et al. Ratiometric monitoring of thorium contamination in natural water using a dual-emission luminescent europium organic framework. Environ. Sci. Technol. 2019, 53, 332–341.
- 51.Wang, Z.; Xu, C.; Lu, Y.X.; et al. Microplasma electrochemistry controlled rapid preparation of fluorescent polydopamine nanoparticles and their application in uranium detection. Chem. Eng. J. 2018, 344, 480–486.
- 52.Zhang, Z.; Zhang, D.; Shi, C.; et al. 3,4-Hydroxypyridinone-modified carbon quantum dot as a highly sensitive and selective fluorescent probe for the rapid detection of uranyl ions. Environ. Sci. Nano 2019, 6, 1457–1465.
- 53.Zheng, Z.J.; Zhang, L.; Wang, L.Z.; et al. Ultrasensitive detection of UO22+ based on dopamine-functionalized MoOx quantum dots. Luminescence 2022, 37, 127–133.
- 54.Ghosh, M.; Swain, K.K.; Singh, P.K. Thioflavin-T incorporated cerium-ATP coordination polymer nanoparticles: A promising system for detection of uranyl ion (UO22+) in aqueous medium. Langmuir 2023, 39, 7017–7028.
- 55.Chen, X.F.; Mei, Q.S.; Yu, L.; et al. Rapid and on-site detection of uranyl ions via ratiometric fluorescence signals based on a smartphone platform. ACS Appl. Mater. Interfaces 2018, 10, 42225–42232.
- 56.Feng, T.T.; Zhao, S.L.; Cao, M.; et al. Highly sensitive and specific uranyl ion detection by a fluorescent sensor containing uranyl-specific recognition sites. Sci. Bull. 2025, 70, 70–77.
- 57.Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; et al. Surface-enhanced raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626.
- 58.Sharma, B.; Frontiera, R.R.; Henry, A.I.; et al. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25.
- 59.Gai, T.; Jiang, J.L.; Wang, S.F.; et al. Photoreduced Ag+/sodium alginate supramolecular hydrogel as a sensitive SERS membrane substrate for rapid detection of uranyl ions. Anal. Chim. Acta 2024, 1316, 342826.
- 60.Gai, T.; Jiang, J.L.; Wang, S.F.; et al. Highly sensitive and selective determination of uranyl ions based on Ag/ Ag2O-COF composite SERS substrate. Talanta 2024, 277, 126407.
- 61.Wang, S.F.; Zou, S.M.; Yang, S.L.; et al. HfO2-wrapped slanted Ag nanorods array as a reusable and sensitive SERS substrate for trace analysis of uranyl compounds. Sens. Actuators. B. Chem. 2018, 265, 539–546.
- 62.Jiang, J.L.; Ma, L.W.; Chen, J.; et al. SERS detection and characterization of uranyl ion sorption on silver nanorods wrapped with Al2O3 layers. Microchim. Acta 2017, 184, 2775–2782.
- 63.He, X.; Wang, S.F.; Liu, Y.; et al. Ultra-sensitive detection of uranyl ions with a specially designed high-efficiency SERS-based microfluidic device. Sci. China Chem. 2019, 62, 1064–1071.
- 64.Wang, N.; Du, J.J.; Li, X.; et al. Magnetic MOF substrates for the rapid and sensitive surface-enhanced raman scattering detection of uranyl. Anal. Chem. 2023, 95, 12956–12963.
- 65.Sun, C.M.; Dong, W.R.; Peng, J.X.; et al. Dual-mode fluorescence-SERS sensor for sensitive and selective detection of uranyl ions based on satellite Fe3O4-Au@CdTe nanostructure. Sens. Actuators. B. Chem. 2020, 325, 128644.
- 66.Wang, S.F.; Jiang, J.L.; Wu, H.X.; et al. Self-assembly of silver nanoparticles as high active surface-enhanced Raman scattering substrate for rapid and trace analysis of uranyl(VI) ions. Spectrochim. Acta A 2017, 180, 23–28.
- 67.Jiang, J.L.; Wang, S.F.; Deng, H.; et al. Rapid and sensitive detection of uranyl ion with citrate-stabilized silver nanoparticles by the surface-enhanced Raman scattering technique. R. Soc. Open Sci. 2018, 5, 181099.
- 68.Yuan, C.; Ge, H.W.; Cao, B.M.; et al. SERS detection of uranyl based on MOF-coated gold nanooctahedron hybrid. Anal. Sci. 2024, 40, 2111–2116.
- 69.Lu, W.; Band, B.S.F.; Yu, Y.; et al. Resonance light scattering and derived techniques in analytical chemistry: Past, present, and future. Microchim. Acta 2007, 158, 29–58.
- 70.Shang, L.; Chen, H.J.; Deng, L.; et al. Enhanced resonance light scattering based on biocatalytic growth of gold nanoparticles for biosensors design. Biosens. Bioelectron. 2008, 23, 1180–1184.
- 71.Zhou, B.; Wang, Y.S.; Yang, H.X.; et al. A sensitive resonance light scattering assay for uranyl ion based on the conformational change of a nuclease-resistant aptamer and gold nanoparticles acting as signal reporters. Microchim. Acta 2014, 181, 1353–1360.
- 72.Shrivastava, A.; Sharma, J.; Soni, V. Various electroanalytical methods for the determination of uranium in different matrices. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 113–129.
- 73.Zhou, Z.P.; Zhou, Y.M.; Liang, X.Z.; et al. Electrochemical sensor for uranium monitoring in natural water based on poly Nile blue modified glassy carbon electrode. J. Solid. State Electrochem. 2022, 26, 1139–1149.
- 74.Ghoreishi, S.M.; Behpour, M.; Mazaheri, S.; et al. Uranyl sensor based on a N,N'-bis(salicylidene)-2-hydroxy-phenylmethanediamine and multiwall carbon nanotube electrode. J. Radioanal. Nucl. Chem. 2012, 293, 201–210.
- 75.Li, Y.J.; Wang, Z.M.; Liu, C.; et al. Graphene oxide modified H4L-ion imprinting electrochemical sensor for the detection of uranyl ions. Z. Anorg. Allg. Chem. 2021, 647, 1914–1920.
- 76.Zhou, Z.P.; Zhou, Y.M.; Liang, X.Z.; et al. Sensitive detection of uranium in water samples using differential pulse adsorptive stripping voltammetry on glassy carbon electrode. J. Radioanal. Nucl. Chem. 2019, 322, 2049–2056.
- 77.Wen, Y.; Sun, Y.C.; Liu, Y.T.; et al. Green synthesis of 2% g-C3N4/SnS2-V3/CQD1 composite photocatalyst from waste plant soot for efficient U(VI) removal: Mechanistic insights. Chem. Eng. J. 2024, 494, 153247.
- 78.Guo, Y.X.; Liu, H.W.; Cao, H.; et al. Complexation of uranyl with benzoic acid in aqueous solution at variable temperatures: Potentiometry, spectrophotometry and DFT calculations. Dalton Trans. 2023, 52, 11265–11271.
- 79.Xie, X.; Tian, Y.; Qin, Z.; et al. Complexation of manganese with glutarimidedioxime: Implication for extraction uranium from seawater. Sci. Rep. 2017, 7, 43503.
- 80.Akl, Z.F.; Ali, T.A. A novel modified screen-printed electrode with triazole surfactant assembled on silver nanoparticles for potentiometric determination of uranium. J. Radioanal. Nucl. Chem. 2017, 314, 1865–1875.
- 81.Yang, M.; Liao, L.F.; Zhang, G.L.; et al. Detection of uranium with a wireless sensing method by using salophen as receptor and magnetic nanoparticles as signal-amplifying tags. J. Radioanal. Nucl. Chem. 2013, 298, 1393–1399.
- 82.Sun, Z.L.; Chen, Z.S.; Tai, X.S.; et al. Uranium extraction from seawater: Methods and challenges. Sci. China Chem. 2025, 68, 3923–3926.
- 83.Wei, G.; Chen, Z.S.; Tai, X.S.; et al. Recent progress of uranium extraction and its catalytic applications. Trans. Tianjin Univ. 2025, 31, 390–402.
- 84.Sun, Z.L.; Liao, Y.; Zhang, Y.Y. Sustainable carbon materials in environmental and energy applications. Sustain. Carbon. Mater. 2025, 1, e007.
- 85.Wang, Z.; Lu, Y.X.; Yuan, H.; et al. Microplasma-assisted rapid synthesis of luminescent nitrogen-doped carbon dots and their application in pH sensing and uranium detection. Nanoscale 2015, 7, 20743–20748.
- 86.Sun, Z.L.; Wang, X.K. Covalent metal-organic frameworks: Emerging star materials for seawater uranium harvesting. Sci. Sin. Chim. 2025, 55, 1–2.
- 87.Liang, L.Y.; Qin, F.P.; Wang, S.; et al. Overview of the materials design and sensing strategies of nanopore devices. Coord. Chem. Rev. 2023, 478, 214998.
- 88.Lu, Y.F.; Yu, L.; Zhang, S.L.; et al. Dual-functional fluorescent metal-organic framework based beads for visual detection and removal of oxytetracycline in real aqueous solution. Appl. Surf. Sci. 2023, 625, 157202.
- 89.Jin, K.; Lee, B.; Park, J. Metal-organic frameworks as a versatile platform for radionuclide management. Coord. Chem. Rev. 2021, 427, 213473.
- 90.Sun, Y.F.; Yu, L.; Wu, K.L.; et al. Non-rare earth doped metal-organic framework for fluorescent detection of uranyl in real seawater. Sens. Actuators. B. Chem. 2025, 436, 137643.
- 91.Wang, Y.; Xing, S.H.; Zhang, X.; et al. A family of functional Ln-organic framework constructed by iodine-substituted aromatic polycarboxylic acid for turn-off sensing of UO22+. Appl. Organomet. Chem. 2019, 33, e4898.
- 92.Cao, X.H.; Sun, Y.B.; Wang, Y.C.; et al. PtRu bimetallic nanoparticles embedded in MOF-derived porous carbons for efficiently electrochemical sensing of uranium. J. Solid. State Electrochem. 2021, 25, 425–433.
- 93.Niu, C.P.; Zhang, C.R.; Cui, W.R.; et al. A conveniently synthesized redox-active fluorescent covalent organic framework for selective detection and adsorption of uranium. J. Hazard. Mater. 2022, 425, 127951.
- 94.Zhen, D.S.; Liu, C.L.; Deng, Q.H.; et al. Novel olefin-linked covalent organic framework with multifunctional group modification for the fluorescence/smartphone detection of uranyl ion. ACS Appl. Mater. Interfaces 2024, 16, 27804–27812.
- 95.Guo, X.T.; Wang, X.Y.; Wen, S.Z.; et al. Silver nanoparticle-grafted amidoxime covalent organic framework: A Highly sensitive and selective SERS substrate for uranium detection in natural water systems. Adv. Funct. Mater. 2025, 35, 2500901.
- 96.Chen, Z.J.; Liu, J.Q.; Wang, W.Y.; et al. Aptamer-regulated colorimetric and electrochemical dual-mode sensor for the detection of uranyl ions utilizing AuNCs@COF composite. Microchim. Acta 2025, 192, 295.
- 97.Lu, H.; Fu, D.; Tai, X.S.; et al. Metal-organic frameworks/covalent-organic frameworks-based materials in organic/inorganic pollutant elimination and CO2 reduction applications. ChemNanoMat 2025, 11, e202500244.
- 98.Qian, H.L.; Wang, Y.; Yan, X.P. Covalent organic frameworks for environmental analysis. Trends Anal. Chem. 2022, 147, 116516.
- 99.Zhao, Y.; Yan, Y.; Wu, Z.; et al. A novel fluorescent covalent organic framework for the selective detection of fluoride ion. J. Mater. Sci. 2022, 57, 13425–13432.
- 100.Leng, R.; Sun, Y.C.; Wang, C.Z.; et al. Design and fabrication of hypercrosslinked covalent organic adsorbents for selective uranium extraction. Environ. Sci. Technol. 2023, 57, 9615–9626.
- 101.Kangas, L.J.; Keller, P.E.; Siciliano, E.R.; et al. The use of artificial neural networks in PVT-based radiation portal monitors. Nucl. Instrum. Methods Phys. Res., Sect. A 2008, 587, 398–412.
- 102.Smith, R.; Spano, T.L.; McDonnell, M.; et al. Interpretable machine learning models classify minerals via spectroscopy. Sci. Rep. 2025, 15, 15807.
- 103.Wabwile, J.M.; Angeyo, H.K.; Massop, A.D. Exploring band-free Raman microspectrometry combined with PCA and MCR-ALS for size-resolved forensic analysis of uranium in aerosols in a model nuclear atmosphere. J. Environ. Radioact. 2023, 270, 107295.
- 104.Jung, Y.E.; Ahn, S.K.; Yim, M.S. Investigation of neural network-based cathode potential monitoring to support nuclear safeguards of electrorefining in pyroprocessing. Nucl. Eng. Technol. 2022, 54, 644–652.
- 105.Bae, J.W.; Hu, J.W. Machine learning framework for predicting uranium enrichments from M400 CZT gamma spectra. Nucl. Instrum. Methods Phys. Res. Sect. A 2024, 1068, 169705.
- 106.Zhang, Y.; Ye, Y.J.; Qiu, J.; et al. Study on quantitative interpretation of uranium spectral gamma-ray logging based on machine learning algorithm. Nucl. Eng. Technol. 2024, 56, 4959–4965.
- 107.Kwan, C.; Ayhan, B.; Stavola, A.; et al. A fast framework for generating radioactive mixture dpectra and its application to remote high-performance mixture identification. Electronics 2025, 14, 1688.
- 108.Wang, Z.H.; Zhou, Y.G.; Zhou, T.; et al. Identification of optimal metal-organic frameworks by machine learning: Structure decomposition, feature integration, and predictive modeling. Comput. Chem. Eng. 2022, 160, 107739.

This work is licensed under a Creative Commons Attribution 4.0 International License.


