- Modified carbon-nitrogen catalysts boost reactant adsorption and product desorption
- Kinetics insights, in situ experiments and density functional theory establish the structure-activity relationship and elucidate catalytic mechanism
- Future research focus on the scalability and stability of carbon-nitrogen catalysts
- Open Access
- Review
Continuously Selective Oxidation of Hydrogen Sulfide over Carbon-Nitrogen Catalysts for Sustainable Environment—A Review
- Hanfeng Ye 1,†,
- Fei Zhao 2,†,
- Shihuan Lyu 3,
- Zhiqi Wen 4,5,
- Dapeng Wu 6,*,
- Can Yang 4,5,*
Author Information
Received: 05 Oct 2025 | Revised: 17 Nov 2025 | Accepted: 24 Nov 2025 | Published: 08 Dec 2025
Highlights
Abstract
Hydrogen sulfide (H2S), a highly toxic and corrosive gas emitted from natural, industrial and agricultural processes, poses severe risks to human health, infrastructure, and environment safety. Although the traditional Claus process has been widely used for H2S removal, thermodynamic constraints lead to the residue of 3–5% H2S in the tail gas, necessitating advanced technologies for further transformation and purification. Continuously selective oxidation of H2S (H2S-CSO) has emerged as a promising technology that can achieve near-complete sulfur recovery and ensure compliance with emission regulations. Carbon-nitrogen catalysts show significant potential to replace metal-based catalysts in the H2S-CSO process due to their tunable electronic structures, cost-effectiveness, and resistance to sulfur poisoning. This review comprehensively summarizes the major milestones in the development of carbon-nitrogen catalysts for H2S-CSO process. We focus on key advances, including construction of active sites (e.g., pyridinic N and metal single atoms), regulation of surface electronic structure (e.g., via elemental doping and defect engineering), the use of supports, optimization of pore structure to facilitate both reactants (H2S and O2) adsorption and sulfur desorption processes. These modifications are critically discussed in relation to catalytic performance and stability, so as to unveil the underlying structure-activity relationships. Despite these advances, review articles dedicated to carbon-nitrogen catalysts for H2S-CSO remain scarce, and this work aims to fill that gap. Current challenges such as sulfur poisoning, SO2 over-oxidation, and catalyst scalability are addressed, along with future directions for the rational design of robust carbon-nitrogen catalysts aimed at sustainable H2S treatment and sulfur resource recovery.
Graphical Abstract
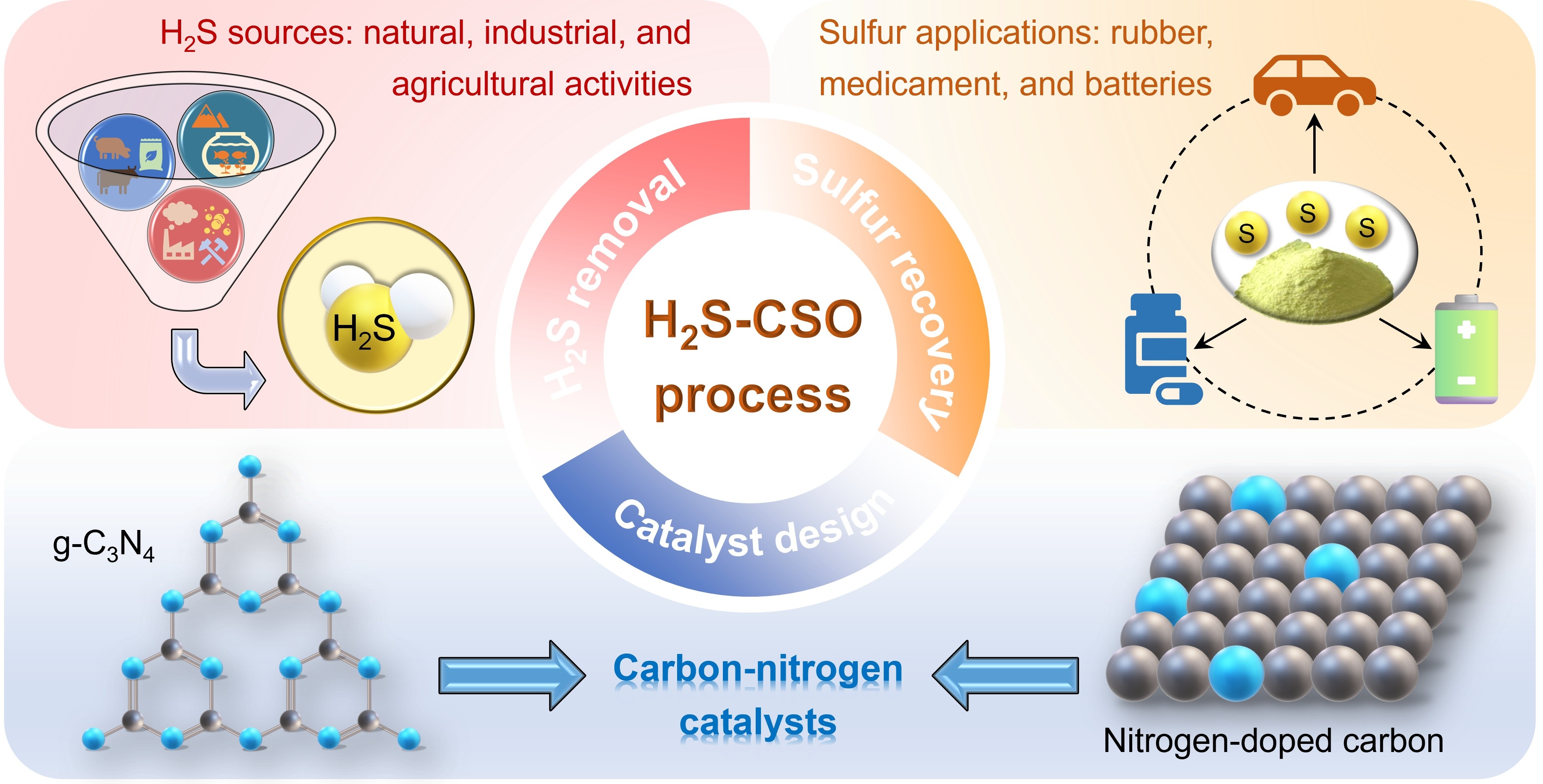
Keywords
hydrogen sulfide | continuously selective oxidation | carbon-nitrogen catalysts | sustainable environment
References
- 1.
Wang, Y.; Chen, X.; Shi, H.; et al. Catalytic reforming of methane with H2S via dynamically stabilized sulfur on transition metal oxides and sulfides. Nat. Catal. 2023, 6, 204–214.
- 2.
Zhao, F.; Wang, C.; Xiong, R.; et al. Crystal Engineering of BiVO4 for Photochemical Sensing of H2S Gas at Ultra-low Concentration. Angew. Chem. Int. Ed. 2023, 62, e202314891.
- 3.
Zhang, C.; Li, A.-Z.; Yuan, B.-J.; et al. Electrochemical valorization of H2S in natural gas to sulfate under mild conditions. Nat. Commun. 2025, 16, 7175.
- 4.
Guidotti, T.L. Hydrogen sulfide: Advances in understanding human toxicity. Int. J. Toxicol. 2010, 29, 569–581.
- 5.
Behl, M.; Yeom, J.; Lineberry, Q.; et al. A regenerable oxide-based H2S adsorbent with nanofibrous morphology. Nat. Nanotechnol. 2012, 7, 810–815.
- 6.
Belmabkhout, Y.; Bhatt, P.M.; Adil, K.; et al. Natural gas upgrading using a fluorinated MOF with tuned H2S and CO2 adsorption selectivity. Nat. Energy 2018, 3, 1059–1066.
- 7.
Duan, C.; Kee, R.J.; Zhu, H.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222.
- 8.
Islamoglu, T.; Chen, Z.; Wasson, M.C.; et al. Metal−Organic Frameworks against Toxic Chemicals. Chem. Rev. 2020, 120, 8130–8160.
- 9.
Yang, H.; Zeng, L.; Wang, J.; et al. Optimizing the electronic configuration of h-BN for boosting the photocatalytic transformation of acid gases under visible light. Env. Sci. Adv. 2024, 3, 97–108.
- 10.
Chiappe, C.; Pomelli, C.S. Hydrogen Sulfide and Ionic Liquids: Absorption, Separation, and Oxidation. Top. Curr. Chem. 2017, 375, 52.
- 11.
Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen Sulfide Capture: From Absorption in Polar Liquids to Oxide, Zeolite, and Metal-Organic Framework Adsorbents and Membranes. Chem. Rev. 2017, 117, 9755–9803.
- 12.
Yang, H.; Dai, Y.; Zeng, L.; et al. H2S adsorbed by bismuth oxychloride under room temperature. Surf. Interfaces 2024, 44, 103663.
- 13.
PiÉPlu, A.; Saur, O.; Lavalley, J.-C.; et al. Claus Catalysis and H2S Selective Oxidation. Catal. Rev. 1998, 40, 409–450.
- 14.
Rodriguez, J.A.; Jirsak, T.; Pérez, M.; et al. Studies on the Behavior of Mixed-Metal Oxides and Desulfurization: Reaction of H2S and SO2 with Cr2O3(0001), MgO(100), and CrxMg1–xO(100). J. Am. Chem. Soc. 2000, 122, 12362–12370.
- 15.
Davydov, A.A.; Marshneva, V.I.; Shepotko, M.L. Metal oxides in hydrogen sulfide oxidation by oxygen and sulfur dioxide. Appl. Catal. A 2003, 244, 93–100.
- 16.
Pandey, R.A.; Biswas, R.; Chakrabarti, T.; et al. Flue Gas Desulfurization: Physicochemical and Biotechnological Approaches. Crit. Rev. Env. Sci. Tec. 2005, 35, 571–622.
- 17.
Zhang, X.; Tang, Y.; Qu, S.; et al. H2S-Selective Catalytic Oxidation: Catalysts and Processes. ACS Catal. 2015, 5, 1053–1067.
- 18.
Steijns, M.; Derks, F.; Verloop, A.; et al. The mechanism of the catalytic oxidation of hydrogen sulfide: II. Kinetics and mechanism of hydrogen sulfide oxidation catalyzed by sulfur. J. Catal. 1976, 42, 87–95.
- 19.
Zhang, X.; Dou, G.; Wang, Z.; et al. Selective catalytic oxidation of H2S over iron oxide supported on alumina-intercalated Laponite clay catalysts. J. Hazard. Mater. 2013, 260, 104–111.
- 20.
Zheng, X.; Li, Y.; Zheng, Y.; et al. Highly Efficient Porous FexCe1–xO2−δ with Three-Dimensional Hierarchical Nanoflower Morphology for H2S-Selective Oxidation. ACS Catal. 2020, 10, 3968–3983.
- 21.
Lei, C.; Zhou, W.; Shen, L.; et al. Enhanced Selective H2S Oxidation Performance on Mo2C-Modified g-C3N4. ACS Sustainable Chem. Eng. 2019, 7, 16257–16263.
- 22.
Mohamadalizadeh, A.; Towfighi, J.; Rashidi, A.; et al. Nanoclays as nano adsorbent for oxidation of H2S into elemental sulfur. Korean J. Chem. Eng. 2011, 28, 1221–1226.
- 23.
Lee, J.D.; Jun, J.H.; Park, N.-K.; et al. A study on selective oxidation of hydrogen sulfide over zeolite-NaX and-KX catalysts. Korean J. Chem. Eng. 2005, 22, 36–41.
- 24.
Zheng, X.; Shen, L.; Lin, F.; et al. Bimetallic Metal–Organic Frameworks MIL-53(xAl–yFe) as Efficient Catalysts for H2S Selective Oxidation. Inorg. Chem. 2022, 61, 3774–3784.
- 25.
Peng, W.-L.; Kan, X.; Chen, W.; et al. Efficiently Selective Oxidation of H2S to Elemental Sulfur over Covalent Triazine Framework Catalysts. ACS Appl. Mater. Interfaces 2021, 13, 34124–34133.
- 26.
Zhang, X.; Wang, Z.; Tang, Y.; et al. Catalytic behaviors of combined oxides derived from Mg/AlxFe1−x–Cl layered double hydroxides for H2S selective oxidation. Catal. Sci. Technol. 2015, 5, 4991–4999.
- 27.
Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A 2001, 212, 17–60.
- 28.
Yang, L.; Wang, S.; Blinn, K.; et al. Enhanced Sulfur and Coking Tolerance of a Mixed Ion Conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3–δ. Science 2009, 326, 126–129.
- 29.
Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269.
- 30.
Boldrin, P.; Ruiz-Trejo, E.; Mermelstein, J.; et al. Strategies for Carbon and Sulfur Tolerant Solid Oxide Fuel Cell Materials, Incorporating Lessons from Heterogeneous Catalysis. Chem. Rev. 2016, 116, 13633–13684.
- 31.
Lin, T.; Chen, I.W.; Liu, F.; et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513.
- 32.
Titirici, M.M.; White, R.J.; Brun, N.; et al. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290.
- 33.
Bai, X.; Hu, P.; Li, A.; et al. Nitrogen-doped amorphous monolayer carbon. Nature 2024, 634, 80–84.
- 34.
Adib, F.; Bagreev, A.; Bandosz, T.J. Adsorption/Oxidation of Hydrogen Sulfide on Nitrogen-Containing Activated Carbons. Langmuir 2000, 16, 1980–1986.
- 35.
Bagreev, A.; Angel Menendez, J.; Dukhno, I.; et al. Bituminous coal-based activated carbons modified with nitrogen as adsorbents of hydrogen sulfide. Carbon 2004, 42, 469–476.
- 36.
Bashkova, S.; Baker, F.S.; Wu, X.; et al. Activated carbon catalyst for selective oxidation of hydrogen sulphide: On the influence of pore structure, surface characteristics, and catalytically-active nitrogen. Carbon 2007, 45, 1354–1363.
- 37.
Brazhnyk, D.V.; Zaitsev, Y.P.; Bacherikova, I.V.; et al. Oxidation of H2S on activated carbon KAU and influence of the surface state. Appl. Catal. B 2007, 70, 557–566.
- 38.
Duong-Viet, C.; Liu, Y.; Ba, H.; et al. Carbon nanotubes containing oxygenated decorating defects as metal-free catalyst for selective oxidation of H2S. Appl. Catal. B 2016, 191, 29–41.
- 39.
Bian, C.; Gao, Q.; Zhang, J.; et al. Impact of pyrone group on H2S catalytic oxidization. Sci. Total Environ. 2019, 695, 133875.
- 40.
Klein, J.; Henning, K.-D. Catalytic oxidation of hydrogen sulphide on activated carbons. Fuel 1984, 63, 1064–1067.
- 41.
Feng, W.; Kwon, S.; Borguet, E.; et al. Adsorption of Hydrogen Sulfide onto Activated Carbon Fibers: Effect of Pore Structure and Surface Chemistry. Environ. Sci. Technol. 2005, 39, 9744–9749.
- 42.
Chen, Q.; Wang, Z.; Long, D.; et al. Role of Pore Structure of Activated Carbon Fibers in the Catalytic Oxidation of H2S. Ind. Eng. Chem. Res. 2010, 49, 3152–3159.
- 43.
Sun, F.; Liu, J.; Chen, H.; et al. Nitrogen-Rich Mesoporous Carbons: Highly Efficient, Regenerable Metal-Free Catalysts for Low-Temperature Oxidation of H2S. ACS Catal. 2013, 3, 862–870.
- 44.
Li, S.; Gu, Q.; Cao, N.; et al. Defect enriched N-doped carbon nanoflakes as robust carbocatalysts for H2S selective oxidation. J. Mater. Chem. A 2020, 8, 8892–8902.
- 45.
Jones, D.I.; Stewart, A.D. Properties of hydrogenated amorphous carbon films and the effects of doping. Philos. Mag. B 1982, 46, 423–434.
- 46.
Kaufman, J.H.; Metin, S.; Saperstein, D.D. Symmetry breaking in nitrogen-doped amorphous carbon: Infrared observation of the Raman-active G and D bands. Phys. Rev. B 1989, 39, 13053–13060.
- 47.
Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Nitrogen- and Boron-Doped Double-Walled Carbon Nanotubes. ACS Nano 2007, 1, 494–500.
- 48.
Bagreev, A.; Bandosz, T.J. Study of Hydrogen Sulfide Adsorption on Activated Carbons Using Inverse Gas Chromatography at Infinite Dilution. J. Phys. Chem. B 2000, 104, 8841–8847.
- 49.
Wu, X.; Schwartz, V.; Overbury, S.H.; et al. Desulfurization of Gaseous Fuels Using Activated Carbons as Catalysts for the Selective Oxidation of Hydrogen Sulfide. Energy Fuels 2005, 19, 1774–1782.
- 50.
Zheng, X.-X.; Shen, L.-J.; Chen, X.-P.; et al. Amino-Modified Fe-Terephthalate Metal–Organic Framework as an Efficient Catalyst for the Selective Oxidation of H2S. Inorg. Chem. 2018, 57, 10081–10089.
- 51.
Yang, C.-M.; Kaneko, K. Nitrogen-Doped Activated Carbon Fiber as an Applicant for NO Adsorbent. J. Colloid Interface Sci. 2002, 255, 236–240.
- 52.
Gong, K.; Du, F.; Xia, Z.; et al. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764.
- 53.
Chizari, K.; Deneuve, A.; Ersen, O.; et al. Nitrogen-Doped Carbon Nanotubes as a Highly Active Metal-Free Catalyst for Selective Oxidation. ChemSusChem 2012, 5, 102–108.
- 54.
Fujisaki, T.; Ikeda, K.; Staykov, A.T.; et al. Density functional theory analysis for H2S adsorption on pyridinic N- and oxidized N-doped graphenes. RSC Adv. 2022, 12, 19955–19964.
- 55.
Zhang, Z.; Zhang, F.; Song, Z.; et al. Oxygen Reduction Reaction on Pyridinic Nitrogen-Functionalized Carbon: Active Site Quantification and Effects of Lewis Basicity. ACS Catal. 2025, 15, 296–309.
- 56.
Guo, D.; Shibuya, R.; Akiba, C.; et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365.
- 57.
Ghasemy, E.; Motejadded, H.B.; rashidi, A.; et al. N-doped CNT nanocatalyst prepared from camphor and urea for gas phase desulfurization: Experimental and DFT study. J. Taiwan Inst. Chem. Eng. 2018, 85, 121–131.
- 58.
Wang, X.; Maeda, K.; Thomas, A.; et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80.
- 59.
Lei, G.; Dai, Z.; Fan, Z.; et al. Porous nanosheets of carbon-conjugated graphitic carbon nitride for the oxidation of H2S to elemental sulfur. Carbon 2019, 155, 204–214.
- 60.
Shen, L.; Lei, G.; Fang, Y.; et al. Polymeric carbon nitride nanomesh as an efficient and durable metal-free catalyst for oxidative desulfurization. Chem. Commun. 2018, 54, 2475–2478.
- 61.
Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; et al. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329.
- 62.
Lei, G.; Qi, S.; Li, H.; et al. Carbon-doped boron nitride nanosheets as an efficient metal-free catalyst for the selective oxidation of H2S. Phys. Chem. Chem. Phys. 2023, 25, 32317–32322.
- 63.
Mi, J.; Liu, F.; Chen, W.; et al. Design of Efficient, Hierarchical Porous Polymers Endowed with Tunable Structural Base Sites for Direct Catalytic Elimination of COS and H2S. ACS Appl. Mater. Interfaces 2019, 11, 29950–29959.
- 64.
Yang, C.; Ye, H.; Byun, J.; et al. N-Rich Carbon Catalysts with Economic Feasibility for the Selective Oxidation of Hydrogen Sulfide to Sulfur. Environ. Sci. Technol. 2020, 54, 12621–12630.
- 65.
Xu, C.; Chen, J.; Li, S.; et al. N-doped honeycomb-like porous carbon derived from biomass as an efficient carbocatalyst for H2S selective oxidation. J. Hazard. Mater. 2021, 403, 123806.
- 66.
Zhang, X.; Xu, C.; Li, S.; et al. N-doped porous carbocatalyst engineering via modulating the crystalline size of ZIF-8 for continuous H2S selective oxidation. Appl. Mater. Today 2021, 25, 101228.
- 67.
Liu, X.; Zhangsun, G.; Zheng, Y.; et al. Hierarchical N-Doped Carbons Endowed with Structural Base Sites toward Highly Selective Adsorption and Catalytic Oxidation of H2S. Ind. Eng. Chem. Res. 2021, 60, 2101–2111.
- 68.
Xu, C.; Gu, Q.; Li, S.; et al. Heteroatom-Doped Monolithic Carbocatalysts with Improved Sulfur Selectivity and Impurity Tolerance for H2S Selective Oxidation. ACS Catal. 2021, 11, 8591–8604.
- 69.
Liu, X.; Zhai, X.; Zhao, Y.; et al. Sulfur modified N-doped carbocatalysts promote the selectivity for H2S selective oxidation. Appl. Catal. B 2025, 362, 124717.
- 70.
Ye, H.; Zhou, M.; Zeng, F.; et al. Functional carbon materials with Lewis pairs for efficiently transforming sulfur compounds from waste to chemical. Appl. Catal. B 2025, 374, 125399.
- 71.
Li, S.; Liu, Y.; Gong, H.; et al. N-Doped 3D Mesoporous Carbon/Carbon Nanotubes Monolithic Catalyst for H2S Selective Oxidation. ACS Appl. Nano Mater. 2019, 2, 3780–3792.
- 72.
Wang, A.; Li, J.; Zhang, T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2, 65–81.
- 73.
Lei, G.; Zhao, W.; Shen, L.; et al. Isolated iron sites embedded in graphitic carbon nitride (g-C3N4) for efficient oxidative desulfurization. Appl. Catal. B 2020, 267, 118663.
- 74.
Lei, G.; Tong, Y.; Shen, L.; et al. Highly Active and Sulfur-Resistant Fe–N4 Sites in Porous Carbon Nitride for the Oxidation of H2S into Elemental Sulfur. Small 2020, 16, 2003904.
- 75.
Lei, G.; Tong, Y.; Shen, L.; et al. Highly Poison-Resistant Single-Atom Co–N4 Active Sites with Superior Operational Stability over 460 h for H2S Catalytic Oxidation. Small 2021, 17, 2104939.
- 76.
Liu, Y.; Song, C.; Wang, Y.; et al. Rational designed Co@N-doped carbon catalyst for high-efficient H2S selective oxidation by regulating electronic structures. Chem. Eng. J. 2020, 401, 126038.
- 77.
Li, Y.; Huang, B.; Li, H.; et al. The effect of coordination states of cobalt on Co-Nx co-doped graphene for selective oxidation of H2S: A DFT study. Diam. Relat. Mat. 2023, 140, 110477.
- 78.
Li, Y.; Yang, Y.; Li, K.; et al. Theoretical analysis of selective catalytic oxidation of H2S on Fe-N3 co-doped graphene. Mol. Catal. 2022, 524, 112318.
- 79.
Tong, Y.; Wei, C.; Wang, J.; et al. Microscopic functionality of FeN4 sites in polymeric carbon nitride for efficient H2S oxidation. Appl. Surf. Sci. 2022, 600, 154011.
- 80.
Ghasemy, E.; Emrooz, H.B.M.; Rashidi, A.; et al. Highly uniform molybdenum oxide loaded N-CNT as a remarkably active and selective nanocatalyst for H2S selective oxidation. Sci. Total Environ. 2020, 711, 134819.
- 81.
Ye, H.; Xing, W.; Zhao, F.; et al. Sabatier Optimal of Mn-N4 Single Atom Catalysts for Selective Oxidative Desulfurization. Angew. Chem. Int. Ed. 2025, 64, e202419630.
- 82.
Lei, G.; Cao, Y.; Zhao, W.; et al. Exfoliation of Graphitic Carbon Nitride for Enhanced Oxidative Desulfurization: A Facile and General Strategy. ACS Sustainable Chem. Eng. 2019, 7, 4941–4950.
- 83.
Zhong, K.; Zhu, X.; Yang, J.; et al. Ultrathin structure of oxygen doped carbon nitride for efficient CO2 photocatalytic reduction. Nanotechnology 2022, 33, 115404.
- 84.
Kamali, F.; Eskandari, M.M.; Rashidi, A.; et al. Nanorod carbon nitride as a carbo catalyst for selective oxidation of hydrogen sulfide to sulfur. J. Hazard. Mater. 2019, 364, 218–226.
- 85.
Lyu, S.; Wu, W.; Xiong, R.; et al. Carbon-Rich carbon nitride nanocatalysts for H2S selective oxidation. J. Catal. 2022, 413, 992–1004.
- 86.
Shen, L.; Lei, G.; Zheng, Y.; et al. Electronic Regulation of Bromophenyl Grafted Metal-Free Carbon Nitride Catalysts for Enhanced Utilization of H2S. ChemCatChem 2021, 13, 2386–2392.
- 87.
Gao, C.; Low, J.; Long, R.; et al. Heterogeneous Single-Atom Photocatalysts: Fundamentals and Applications. Chem. Rev. 2020, 120, 12175–12216.
- 88.
Lyu, S.; Wang, J.; Zhou, Y.; et al. Structural Lithium Incorporated with the Crystalline Poly(Triazine Imide) Frameworks for Selective Catalytic Oxidative Desulfurization. Adv. Funct. Mater. 2024, 34, 2310286.
- 89.
Shinkarev, V.V.; Glushenkov, A.M.; Kuvshinov, D.G.; et al. Nanofibrous carbon with herringbone structure as an effective catalyst of the H2S selective oxidation. Carbon 2010, 48, 2004–2012.
- 90.
Truong-Huu, T.; Duong-Viet, C.; Duong-The, H.; et al. Radiofrequency-driven selective oxidation of H2S on hierarchical metal-free catalyst containing defects. Appl. Catal. A 2021, 620, 118171.
- 91.
Housseinou, B.; Cuong, D.-V.; Liu, Y.; et al. Nitrogen-doped carbon nanotube spheres as metal-free catalysts for the partial oxidation of H2S. C.R. Chim. 2016, 19, 1303–1309.
- 92.
Duong-Viet, C.; Ba, H.; Liu, Y.; et al. Nitrogen-doped carbon nanotubes on silicon carbide as a metal-free catalyst. Chin. J. Catal. 2014, 35, 906–913.
- 93.
Duong-Viet, C.; Nhut, J.-M.; Truong-Huu, T.; et al. A nitrogen-doped carbon-coated silicon carbide as a robust and highly efficient metal-free catalyst for sour gas desulfurization in the presence of aromatics as contaminants. Catal. Sci. Technol. 2020, 10, 5487–5500.
- 94.
Cuong, D.-V.; Truong-Phuoc, L.; Tran-Thanh, T.; et al. Nitrogen-doped carbon nanotubes decorated silicon carbide as a metal-free catalyst for partial oxidation of H2S. Appl. Catal. A 2014, 482, 397–406.
- 95.
Wang, W.; Duong-Viet, C.; Truong-Phuoc, L.; et al. Improving catalytic performance via induction heating: Selective oxidation of H2S on a nitrogen-doped carbon catalyst as a model reaction. New J. Chem. 2023, 47, 1105–1116.
- 96.
Duong-Viet, C.; Nhut, J.-M.; Truong-Huu, T.; et al. Tailoring Properties of Metal-Free Catalysts for the Highly Efficient Desulfurization of Sour Gases under Harsh Conditions Catalysts [Online], 2021, p. 226.
- 97.
Wang, T.; Liu, X.; Shan, L.; et al. Manipulation of Waste-Activated Carbon Absorbers as Efficient Carbocatalysts for H2S Selective Oxidation. Ind. Eng. Chem. Res. 2024, 63, 4380–4389.
- 98.
Liu, Y.; Duong-Viet, C.; Luo, J.; et al. One-Pot Synthesis of a Nitrogen-Doped Carbon Composite by Electrospinning as a Metal-Free Catalyst for Oxidation of H2S to Sulfur. ChemCatChem 2015, 7, 2957–2964.
- 99.
Liu, X.; Zhao, M.; Liu, D.; et al. Boosting catalytic oxidation of H2S over activated carbon optimized by the synergistic effect of rich defects and nitrogen sites. Surf. Interfaces 2025, 68, 106672.
- 100.
Liu, X.; Shan, L.; Sun, X.; et al. Reusable salt-template strategy for synthesis of porous nitrogen-rich carbon boosts H2S selective oxidation. Green Energy Environ. 2024, 9, 1866–1877.
- 101.
Liu, X.; Zhao, Y.; Ma, J.; et al. Defects mediate the active N sites in N-doped carbon catalyst for efficiently catalyzing H2S to element sulfur. Appl. Catal. B 2026, 380, 125718.
- 102.
Kan, X.; Chen, X.; Chen, W.; et al. Nitrogen-Decorated, Ordered Mesoporous Carbon Spheres as High-Efficient Catalysts for Selective Capture and Oxidation of H2S. ACS Sustainable Chem. Eng. 2019, 7, 7609–7618.
- 103.
Lei, G.; Fan, Z.; Hou, Y.; et al. Facile template-free synthesis of 3D cluster-like nitrogen-doped mesoporous carbon as metal-free catalyst for selective oxidation of H2S. J. Environ. Chem. Eng. 2023, 11, 109095.
- 104.
Kim, S.Y.; Phule, A.D.; Yang, J.H.; et al. Improving H2S remediation efficiency through metal-free biochar modification: Nitrogen introduction and mesopore formation. J. Anal. Appl. Pyrolysis 2024, 183, 106822.
- 105.
Yang, J.; Cui, S.; Zhao, F.; et al. Waste to Wealth: Discarded Cigarette Butt-Derived Metal-Free N-Rich Carbon Catalysts for the Selective Catalytic Oxidation of Hydrogen Sulfide to Sulfur. Environ. Sci. Technol. 2024, 58, 20267–20276.
- 106.
Li, S.; Fu, H.; Zhang, X.; et al. Hierarchically porous, N-defect enriched C-nanosheets boost the H2S selective oxidation to elemental sulfur. Appl. Catal. B 2024, 343, 123505.
- 107.
Li, H.; Zhao, J.; Pan, S.; et al. Template-free fabrication of nitrogen-doped mesoporous carbon catalysts with high water resistance for selective catalytic oxidation of H2S. Appl. Surf. Sci. 2025, 693, 162703.
- 108.
Shan, L.; Liu, X.; Zhao, Y.; et al. Hierarchical Porous N-Doped Carbon Particles Derived from ZIF-8 as Highly Efficient H2S Selective Oxidation Catalysts. ACS Appl. Mater. Interfaces 2024, 16, 23314–23324.
- 109.
Chung, W.J.; Griebel, J.J.; Kim, E.T.; et al. The Use of Elemental Sulfur as An Alternative Feedstock for Polymeric Materials. Nat. Chem. 2013, 5, 518–524.
- 110.
Boyd, D.A. Sulfur and Its Role In Modern Materials Science. Angew. Chem. Int. Ed. 2016, 55, 15486–15502.

This work is licensed under a Creative Commons Attribution 4.0 International License.


