- Frequent surface-water-groundwater interactions across the watershed basin
- Microbial nitrogen cycling roadmap indicating N-transformation hotspots
- Temperature, DO, and pH as key modulators of microbial co-occurrence networks
- Open Access
- Article
Microbial Community Dynamics in the Surface Water-Groundwater Interaction Zone of the Upper Miyun Reservoir Basin
- Zhaoxin Li 1,2,
- Wenzhi Zhang 1,3,
- Junxiong Huang 1,*,
- Zhaoyong Bian 2,*,
- Lei Li 1,
- Wanlai Xue 1,
- Wei Xiu 3,*
Author Information
Received: 04 Nov 2025 | Revised: 09 Dec 2025 | Accepted: 16 Dec 2025 | Published: 23 Dec 2025
Highlights
Abstract
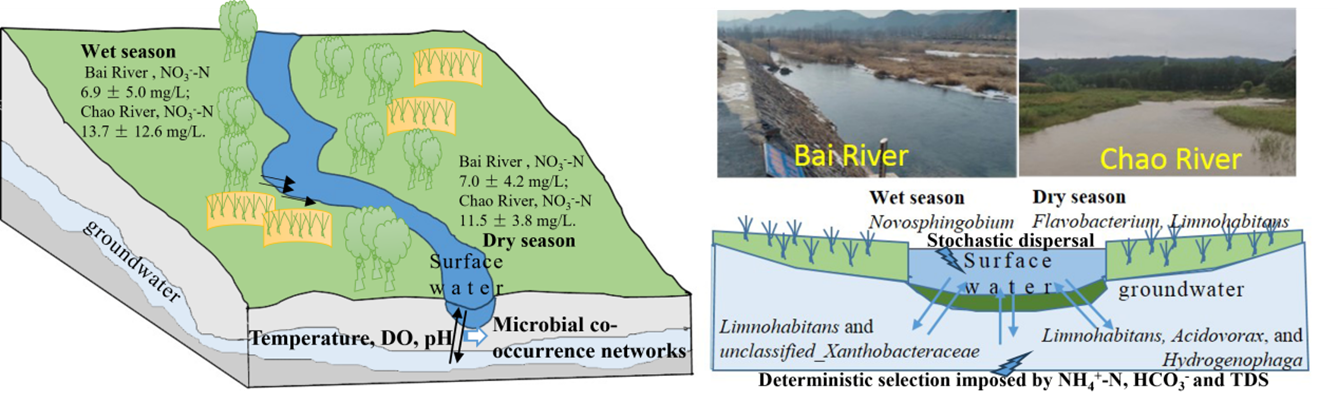
Nitrogen (N) fluxes delivered from river inflows to reservoirs are critical for regulating nutrient dynamics and sustaining water quality in drinking-water source regions. In this study, we investigated the surface-water-groundwater interaction zone within the Chao-Bai River catchment, the primary inflow area feeding the Miyun Reservoir, which is the main water supply for Beijing. Our objective was to characterize the spatiotemporal variability of N species, microbial community compositions, assembly processes, and functional taxa, and to clarify their respective contributions to N cycling. Nitrate-N, which represented 88 ± 9% of total N, was consistently higher in the Chao River (wet season: 13.7 ± 12.6 mg/L; dry season: 11.5 ± 3.8 mg/L) than in the Bai River (wet season: 6.9 ± 5.0 mg/L; dry season: 7.0 ± 4.2 mg/L). Microbial assemblies differed markedly across hydrological compartments and seasons. During the wet season, Novosphingobium dominated surface waters, whereas Limnohabitans and unclassified_Xanthobacteraceae were prevalent in groundwater, and Flavobacterium was abundant in sediments. In the dry season, surface waters were co-dominated by Flavobacterium and Limnohabitans, while Limnohabitans, Acidovorax, and Hydrogenophaga were characteristic of groundwater communities. Temperature, dissolved oxygen, and pH emerged as the principal environmental drivers structuring microbial interactions. Stochastic processes primarily governed microbial community assembly in surface waters and sediments, whereas deterministic selection exerted stronger control in groundwater. Overall, the results demonstrate that hydrological connectivity and microbial dynamics interactively regulate N fluxes and transformation pathways at the river-reservoir interface. These insights provide a mechanistic foundation for improving nutrient management and protecting drinking-water sources in reservoir ecosystems.
Graphical Abstract
Keywords
surface water-groundwater interface | nitrogen cycling | microbial community | environmental determinants | community assembly processes
References
- 1.
Cai, Y.; Feng, M.; Zhang, T. Review of Distribution of Nitrogen and Phosphorus in Riparian Zones of Chinese Inland Water Bodies. Acta Ecol. Sin. 2022, 42, 583–592.
- 2.
Sun, M.; Zhang, L.; Yang, R.; et al. Water Resource Dynamics and Protection Strategies for Inland Lakes: A Case Study of Hongjiannao Lake. J. Environ. Manag. 2024, 355, 120462.
- 3.
Ning, H.; Jiang, W.; Sheng, Y.; et al. Comprehensive Evaluation of Nitrogen Contamination in Water Ecosystems of the Miyun Reservoir Watershed, Northern China: Distribution, Source Apportionment and Risk Assessment. Environ. Geochem. Health 2024, 46, 278.
- 4.
Zhang, X.; Qi, Y.; Li, H.; et al. Assessing the Response of Non-Point Source Nitrogen Pollution to Land Use Change Based on SWAT Model. Ecol. Indic. 2024, 158, 111391.
- 5.
Xu, W.; Cai, Y.; Rong, Q.; et al. Agricultural Non-Point Source Pollution Management in a Reservoir Watershed Based on Ecological Network Analysis of Soil Nitrogen Cycling. Environ. Sci. Pollut. Res. 2018, 25, 9071–9084.
- 6.
Sarah, H.L.; Martin, B.; Robin, G.; et al. Connecting Diverse Disciplines to Improve Understanding of Surface Water-Groundwater Interactions. J. Hydrol. X 2022, 17, 100141.
- 7.
Ren, Y.; Xu, Z.; Zhang, X.; et al. Nitrogen Pollution and Source Identification of Urban Ecosystem Surface Water in Beijing. Front. Environ. Sci. Eng. 2014, 8, 106–116.
- 8.
Andrew, C.P.; Richard, I.; Stuart, B.; et al. Source Partitioning Using Stable Isotopes: Coping with Too Much Variation. PLoS ONE 2010, 5, e9672.
- 9.
Stein, L.Y.; Klotz, M.G. The Nitrogen Cycle. Curr. Biol. 2016, 26, R94–R98.
- 10.
Cao, Q.; Wang, H.; Chen, X.; et al. Composition and Distribution of Microbial Communities in Natural River Wetlands and Corresponding Constructed Wetlands. Ecol. Eng. 2017, 98, 40–48.
- 11.
Stonedahl, S.H.; Sawyer, A.H.; Stonedahl, F.; et al. Effect of Heterogeneous Sediment Distributions on Hyporheic Flow in Physical and Numerical Models. Groundwater 2018, 56, 934–946.
- 12.
Cheng, B.; Jiang, B.; Zhang, R.; et al. Variation Analysis of Long-term TN Concentration and Influencing Factors in Miyun Reservoir in China. Glob. NEST J. 2023, 25, 138–147.
- 13.
Bao, Y.; Zhang, D.; Wang, Y.; et al. Analysis of Nitrogen Migration and Transformation in the Typical Deep and Large Reservoir of the Lancang River—Evidence from Nitrogen and Oxygen Isotopes. J. Hydrol. 2024, 640, 131701.
- 14.
He, D.; Xie, X.; Liu, T.; et al. Fast Migrations of Nitrogen and Phosphorus Are Driven by Microorganism in Freshwater Lake Sediments. J. Soils Sediments 2024, 24, 1391–1401.
- 15.
He, Z.; Cai, J.; Ni, Z.; et al. Seasonal Characteristics of Nitrogen Sources from Different Ways and Its Contribution to Water Nitrogen in Lake Erhai. Acta Sci. Circumstantiae 2018, 38, 1939–1948.
- 16.
Wu, Y.; Wang, J.; Liu, Z.; et al. Seasonal Nitrate Input Drives the Spatiotemporal Variability of Regional Surface Water-Groundwater Interactions, Nitrate Sources and Transformations. J. Hydrol. 2025, 655, 132973.
- 17.
Zhao, Z.; Chen, Y.; Ye, C.; et al. Linkage between Nitrogen Loss, River Transport, Lake Accumulation and Water Quality Properties in Plain River Network Basin. J. Environ. Sci. 2025, 157, 65–76.
- 18.
Dahan, O.; Babad, A.; Lazarovitch, N.; et al. Nitrate Leaching from Intensive Organic Farms to Groundwater. Hydrol. Earth Syst. Sci. 2014, 18, 333–341.
- 19.
Han, Y.; Zhou, J.; Xia, F.; et al. Distribution of Nitrate Nitrogen and Oxygen Isotopes along the Hydrological Path in Alpine Forests. China Environ. Sci. 2025, 45, 935–942.
- 20.
Xu, N.; Tan, G.; Wang, H.; et al. Effect of Biochar Additions to Soil on Nitrogen Leaching, Microbial Biomass and Bacterial Community Structure. Eur. J. Soil Biol. 2016, 74, 1–8.
- 21.
Wickham, H. ggplot2. WIREs Comput. Stat. 2011, 3, 180–185.
- 22.
Available online: https://CRAN.R-project.org/package=ggsignif (accessed on 12 October 2024).
- 23.
Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 18 April 2025).
- 24.
Available online: https://cran.r-project.org/web/packages/Hmisc/index.html (accessed on 12 March 2025).
- 25.
Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 22 January 2025).
- 26.
Available online: https://github.com/DaliangNing/NST (accessed on 6 March 2025).
- 27.
Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the International AAAI Conference on Web and Social Media, Media San Jose, CA, USA, 17–20 May 2009; pp. 361–362.
- 28.
Wen, T.; Xie, P.; Yang, S.; et al. ggClusterNet: An R Package for Microbiome Network Analysis and Modularity-Based Multiple Network Layouts. iMeta 2022, 1, e32.
- 29.
Huang, W.; Chen, X.; Wang, K.; et al. Comparison among the Microbial Communities in the Lake, Lake Wetland, and Estuary Sediments of a Plain River Network. MicrobiologyOpen 2019, 8, e00644.
- 30.
Zhang, T.; Wang, W.; Leng, Y.; et al. Bacterial Diversity and Vertical Distribution Patterns in Sandy Sediments: A Study on the Bacterial Community Structure Based on Environmental Factors in Tributaries of the Yangtze River. Microorganisms 2024, 12, 1178.
- 31.
Zhao, Z.; Zhao, R.; Qiu, X.; et al. Structural Diversity of Bacterial Communities and Its Relation to Environmental Factors in the Surface Sediments from Main Stream of Qingshui River. Water 2022, 14, 3356.
- 32.
Pélabon, C.; Hilde, C.H.; Einum, S.; et al. On the Use of the Coefficient of Variation to Quantify and Compare Trait Variation. Evol. Lett. 2020, 4, 180–188.
- 33.
Zhao, Y.-P. Characteristics and Influencing Factors of Soil Nitrogen-Cycling Microbial Communities under Different Land-Use Types. Ph.D. Thesis, Southwest University, Chongqing, China, 2024.
- 34.
Sloan, W.T.; Lunn, M.; Woodcock, S.; et al. Quantifying the Roles of Immigration and Chance in Shaping Prokaryote Community Structure. Environ. Microbiol. 2006, 8, 732–740.
- 35.
Chen, W.; Ren, K.; Isabwe, A.; et al. Stochastic Processes Shape Microeukaryotic Community Assembly in a Subtropical River across Wet and Dry Seasons. Microbiome 2019, 7, 138.
- 36.
Ning, D.; Deng, Y.; Tiedje, J.M.; et al. A General Framework for Quantitatively Assessing Ecological Stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898.
- 37.
Takeuchi, M.; Hamana, K.; Hiraishi, A. Proposal of the Genus Sphingomonas Sensu Stricto and Three New Genera, Sphingobium, Novosphingobium and Sphingopyxis, on the Basis of Phylogenetic and Chemotaxonomic Analyses. Int. J. Syst. Evol. Microbiol. 2001, 51, 1405–1417.
- 38.
Liu, Y.; Pei, T.; Du, J.; et al. Comparative Genomic Analysis of the Genus Novosphingobium and the Description of Two Novel Species Novosphingobium aerophilum sp. nov. and Novosphingobium jiangmenense sp. nov. Syst. Appl. Microbiol. 2021, 44, 126202.
- 39.
Li, Y.; Sun, Y.; Zhang, H.; et al. The Responses of Bacterial Community and N₂O Emission to Nitrogen Input in Lake Sediment: Estrogen as a Co-pollutant. Environ. Res. 2019, 179, 108769.
- 40.
Huang, S.; Zhang, B.; Zhao, Z.; et al. Metagenomic Analysis Reveals the Responses of Microbial Communities and Nitrogen Metabolic Pathways to Polystyrene Micro (nano) Plastics in Activated Sludge Systems. Water Res. 2023, 241, 120161.
- 41.
Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional Ecology of Free-Living Nitrogen Fixation: A Contemporary Perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512.
- 42.
Ramond, J.-B.; Jordaan, K.; Díez, B.; et al. Microbial Biogeochemical Cycling of Nitrogen in Arid Ecosystems. Microbiol. Mol. Biol. Rev. 2022, 86, e00109-21.
- 43.
Heylen, K.; Lebbe, L.; De Vos, P. Acidovorax caeni sp. nov., a Denitrifying Species with Genetically Diverse Isolates from Activated Sludge. Int. J. Syst. Evol. Microbiol. 2008, 58, 73–77.
- 44.
Willems, A.; Gillis, M. Hydrogenophaga. In Bergey’s Manual of Systematics of Archaea and Bacteria; Tindall, B.J., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–15.
- 45.
Kasalický, V.; Jezbera, J.; Hahn, M.W.; et al. The Diversity of the Limnohabitans Genus, an Important Group of Freshwater Bacterioplankton, by Characterization of 35 Isolated Strains. PLoS ONE 2013, 8, e58209.
- 46.
Su, J.-F.; Zhang, K.; Huang, T.-L.; et al. Heterotrophic Nitrification and Aerobic Denitrification at Low Nutrient Conditions by a Newly Isolated Bacterium, Acinetobacter sp. SYF26. Microbiology 2015, 161, 829–837.
- 47.
Giri, S.; Pati, B. A Comparative Study on Phyllosphere Nitrogen Fixation by Newly Isolated Corynebacterium sp. & Flavobacterium sp. and Their Potentialities as Biofertilizer. Acta Microbiol. Immunol. Hung. 2004, 51, 47–56.
- 48.
Lima, F.; Hadzibeganovic, T.; Stauffer, D. Evolution of Tag-Based Cooperation on Erdős–Rényi Random Graphs. Int. J. Mod. Phys. C 2014, 25, 1450006.
- 49.
Guseva, K.; Darcy, S.; Simon, E.; et al. From Diversity to Complexity: Microbial Networks in Soils. Soil Biol. Biochem. 2022, 169, 108604.
- 50.
Chen, Y.; Li, Y.; Qiu, T.; et al. High Nitrogen Fertilizer Input Enhanced the Microbial Network Complexity in the Paddy Soil. Soil Ecol. Lett. 2024, 6, 230205.
- 51.
Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.; et al. Keystone Taxa as Drivers of Microbiome Structure and Functioning. Nat. Rev. Microbiol. 2018, 16, 567–576.
- 52.
Spain, A.M.; Forsberg, C.W.; Krumholz, L.R.; et al. Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015.
- 53.
Anderson, C.R.; Condron, L.M.; Clough, T.J.; et al. Biochar Induced Soil Microbial Community Change: Implications for Biogeochemical Cycling of Carbon, Nitrogen and Phosphorus. Pedobiologia 2011, 54, 309–320.
- 54.
Yu, M.; Su, W.Q.; Huang, L.; et al. Bacterial Community Structure and Putative Nitrogen-Cycling Functional Traits Along a Charosphere Gradient under Waterlogged Conditions. Soil Biol. Biochem. 2021, 162, 108420.
- 55.
Qiu, L.; Fan, M.; Li, Y.; et al. Soil Phosphorus Drives Variation in Diazotrophic Communities in a Subtropical Nitrogen-Rich Forest. For. Ecol. Manag. 2023, 544, 121164.

This work is licensed under a Creative Commons Attribution 4.0 International License.


