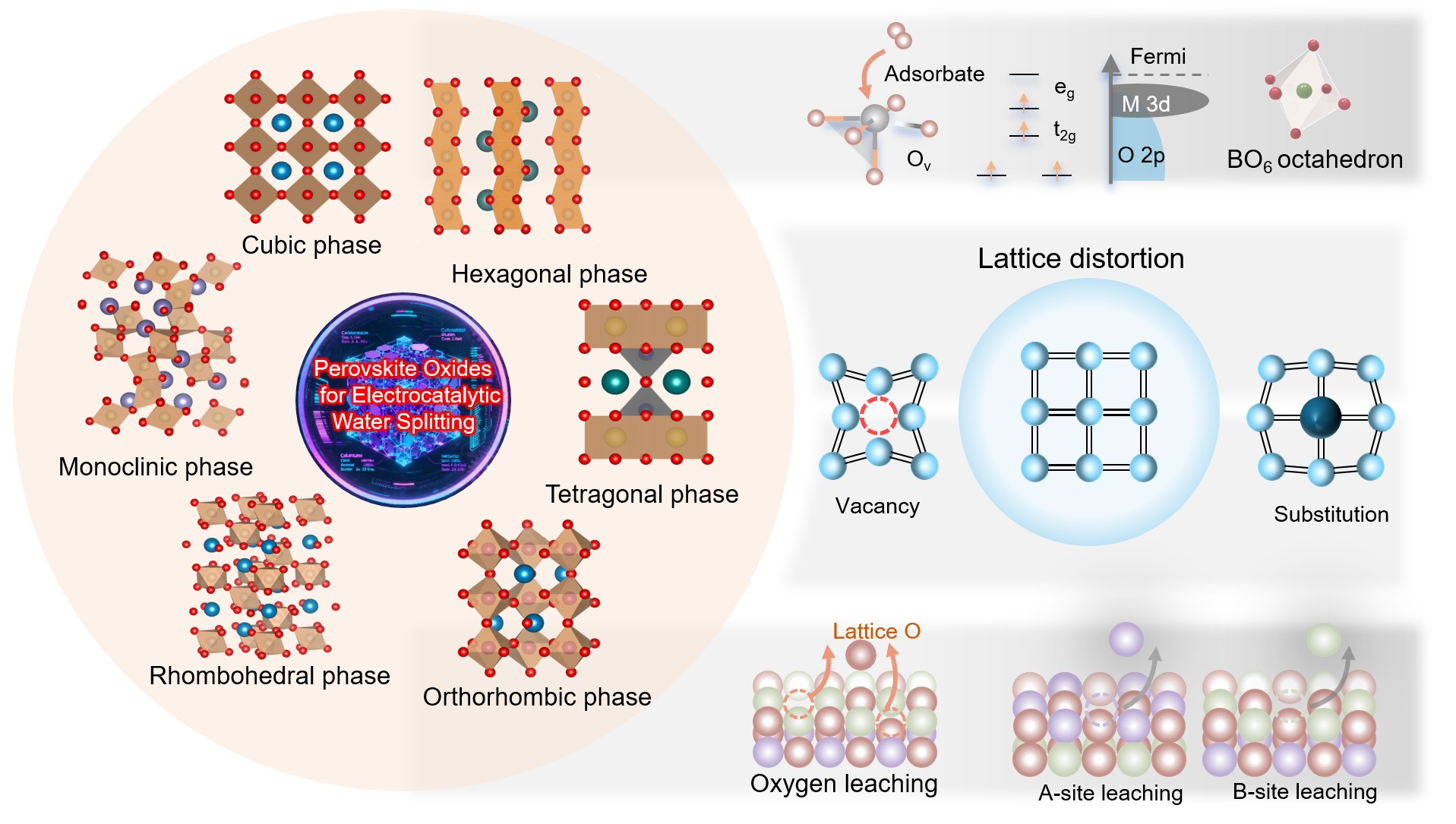
Electrocatalytic water splitting is a key technology for sustainable hydrogen production, yet the sluggish kinetics of the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) hinder its efficiency. Perovskite oxides (ABO3) have emerged as promising catalysts owing to their tunable crystal structures, chemical stability, and abundant active sites. While the crystal phase of perovskite oxides is crucial in determining catalytic activity, charge transport, and reaction energetics, the complex interplay between phase transitions, lattice distortions, oxygen vacancy distribution, and electronic structure remains underexplored. This review systematically examines the structural variations across six primary crystal phases of perovskite oxides, including cubic, hexagonal, tetragonal, orthorhombic, rhombohedral, monoclinic, and their effects on catalytic performance. It explores how lattice symmetry, oxygen vacancy distribution, and electronic properties influence reaction pathways and catalytic efficiency in water splitting. Additionally, the review discusses the role of phase transitions, coordination environment adjustments, and defect engineering in optimizing electrocatalytic behavior. Perovskite oxides are categorized by their A-site and B-site metal ion compositions, providing a comprehensive analysis of how these structural variations influence electrocatalytic mechanisms. The insights gained from this review offer crucial guidance for advancing the design of high-performance perovskite oxide catalysts for sustainable water electrolysis.
- Open Access
- Review
Crystallographic Symmetry—Governed Oxygen-Defect Chemistry in Perovskite Oxides for Electrocatalytic Water Splitting
- Linai Zhou 1,†,
- Jiamu Feng 1,†,
- Kean Zhu 1,†,
- Lingfeng Gao 1,*,
- Weilin Xu 1,
- Hui Wang 2,*,
- Jun Wan 1,*
Author Information
Received: 11 Dec 2025 | Revised: 30 Dec 2025 | Accepted: 08 Jan 2026 | Published: 20 Jan 2026
Abstract
Graphical Abstract
Keywords
perovskite oxides | crystal phase engineering | cation modulation | electronic orbital configuration | electrocatalytic water splitting
References
- 1.
Wang, L.; Yuan, Z.-Y. Elevating Electrode Catalyst Stabilization for Long-Term Intermittent Alkaline Seawater Electrolysis. Smart Mater. Devices 2025, 1, 202527.
- 2.
Zheng, Y.; Ma, M.; Shao, H. Recent Advances in Efficient and Scalable Solar Hydrogen Production through Water Splitting. Carbon Neutrality 2023, 2, 23.
- 3.
Cai, J.; Wu, Z.; Wang, S.; et al. Exploring Advanced Microwave Strategy for the Synthesis of Two-Dimensional Energy Materials. Appl. Phys. Rev. 2024, 11, 041320–041331.
- 4.
Li, N.; Cheng, C.; Wang, Y.; et al. Layered Perovskite Materials for Photocatalytic Overall Water Splitting: Recent Advances, Enhanced Strategies, and Future Challenges. EcoEnergy 2025, 3, e70023.
- 5.
Zhou, P.; Zhao, X.; Song, Y.; et al. pH-Dependent Electrochemical Oxidation of 5-Hydroxymethylfurfural: Reaction Mechanism, Catalyst Design, and Reactor Design Across Alkaline to Acidic Media. Smart Mol. 2025, e70027. https://doi.org/10.1002/smo2.70027.
- 6.
Moon, J.; Beker, W.; Siek, M.; et al. Active Learning Guides Discovery of a Champion Four-Metal Perovskite Oxide for Oxygen Evolution Electrocatalysis. Nat. Mater. 2024, 23, 108–115.
- 7.
Jiang, C.; He, H.; Guo, H.; et al. Transfer Learning Guided Discovery of Efficient Perovskite Oxide for Alkaline Water Oxidation. Nat. Commun. 2024, 15, 6301.
- 8.
Rong, C.; Dastafkan, K.; Wang, Y.; et al. Breaking the Activity and Stability Bottlenecks of Electrocatalysts for Oxygen Evolution Reactions in Acids. Adv. Mater. 2023, 35, 2211884.
- 9.
Xiao, Z.; Xiao, X.; Kong, L.B.; et al. Preparation of MXene-Based Hybrids and Their Application in Neuromorphic Devices. Int. J. Extrem. Manuf. 2024, 6, 022006.
- 10.
Wang, Y.; Wang, L.; Zhang, K.; et al. Electrocatalytic Water Splitting over Perovskite Oxide Catalysts. Chin. J. Catal. 2023, 50, 109–125.
- 11.
Sun, X.; Yuan, Y.; Liu, S.; et al. Recent Advances in Perovskite Oxides for Oxygen Evolution Reaction: Structures, Mechanisms, and Strategies for Performance Enhancement. Adv. Funct. Mater. 2025, 35, 2416705.
- 12.
Bai, Z.; Wang, Z.; Wang, T.; et al. Cation-Vacancy Engineering Modulated Perovskite Oxide for Boosting Electrocatalytic Conversion of Polysulfides. Adv. Funct. Mater. 2025, 35, 2419105.
- 13.
Li, Z.; Li, M.; Zhu, Z. Perovskite Cathode Materials for Low-Temperature Solid Oxide Fuel Cells: Fundamentals to Optimization. Electrochem. Energy Rev. 2022, 5, 263–311.
- 14.
Sun, H.; Xu, X.; Song, Y.; et al. Recent Progress in Sr2Fe1.5Mo0.5O6−δ-Based Multifunctional Materials for Energy Conversion and Storage. Adv. Funct. Mater. 2024, 34, 2411622.
- 15.
Li, K.; Liu, Z.; Han, X.; et al. Local Defect Engineering Tailored Core-Shell Crystalline Perovskite Oxide@Amorphous Hydroxide Heterostructures Activating Dual-Active Sites for High-Performance Zinc-Air Battery Cathodes. J. Energy Chem. 2025, 111, 190–201.
- 16.
Wang, X.; Yuan, Z.-Y. Key Requirements for Photocatalysts and Reactor Architectures Toward Large-Scale Hydrogen Generation. Smart Mater. Devices 2025, 1, 202528.
- 17.
Sun, Y.; Li, R.; Chen, X.; et al. A-Site Management Prompts the Dynamic Reconstructed Active Phase of Perovskite Oxide OER Catalysts. Adv. Energy Mater. 2021, 11, 2003755.
- 18.
Wan, J.; Wu, Z.; Fang, G.; et al. Microwave-Assisted Exploration of the Electron Configuration-Dependent Electrocatalytic Urea Oxidation Activity of 2D Porous NiCo2O4 Spinel. J. Energy Chem. 2024, 91, 226–235.
- 19.
Chu, L.; Shen, H.; Wei, H.; et al. Morphology Engineering of ZnO Micro/Nanostructures Under Mild Conditions for Optoelectronic Application. Int. J. Miner. Metall. Mater. 2025, 32, 498–503.
- 20.
Du, D.; Zheng, R.; He, M.; et al. A-Site Cationic Defects Induced Electronic Structure Regulation of LaMnO3 Perovskite Boosts Oxygen Electrode Reactions in Aprotic Lithium–Oxygen Batteries. Energy Storage Mater. 2021, 43, 293–304.
- 21.
Wei, L.; Dai, J.; Qin, S.; et al. Two-Dimensional Materials for High-Current-Density Seawater Electrolysis. Green Chem. 2025, 27, 8755–8776.
- 22.
Kadi, H.; Bo, L.; Ziqi, T.; et al. Multiscale Simulation in Fuel Cell and Electrolyzer Systems: A Review of Methods, Applications, and Future Prospects. Sustain. Eng. Novit. 2025, 1, 5.
- 23.
Tian, H.; Zhang, H.; Zhu, Z.; et al. Near-Unity Solar Reflectance and Mid-Infrared Transparency via Microwave-Engineered 2D Y2O3 for Passive Radiative Cooling. J. Mater. Chem. A 2025, 13, 39330–39339.
- 24.
Yao, Q.; Liu, P.; Yang, F.; et al. Ferroelectric Polarization in Bi0.9Dy0.1FeO3/g-C3N4 Z-Scheme Heterojunction Boosts Photocatalytic Hydrogen Evolution. Sci. China Mater. 2024, 67, 3160–3167.
- 25.
Wang, S.; Zhang, T.; Zhang, H.; et al. Multifunctional Polyimide Performance Prediction Based on Explainable Machine Learning. Smart Mol. 2025, 3, e70020.
- 26.
Li, K.; Li, Y.; Han, X.; et al. Quenching-Induced Surface Reconstruction of Perovskite Oxides Activating Bifunctional Sites Towards Oxygen Electrodes for Recharge Zinc–Air Batteries. Energy Storage Mater. 2025, 78, 104289.
- 27.
Hao, Y.-R.; Xue, H.; Sun, J.; et al. Achieving Superior Oxygen Evolution of Perovskite via Phase Transition and Electrochemical Reconstruction Strategy. Energy Environ. Sci. 2024, 17, 4044–4054.
- 28.
Ke, L.; Pang, S.; Long, C.; et al. Quenching-Induced Surface Reconstruction of Perovskite Oxide for Rapid and Durable Oxygen Catalysis. Chem. Eng. J. 2023, 463, 142509.
- 29.
Qin, S.; Dai, J.; Wang, M.; et al. Unleashing the Potential of Metastable Materials in Electrocatalytic Water Splitting. ACS Mater. Lett. 2025, 7, 524–543.
- 30.
Xu, K.; Li, M.; Qin, A.; et al. Temperature Self-Regulation, Energy Storage, and Fire Safety Intelligent Wood for Safe and Energy-Efficient Buildings. EcoEnergy 2025, 3, e70019.
- 31.
Liu, Y.; Liu, J.; Hao, L.; et al. Incorporating Crystalline Smart Materials to Fabricate 4D Printed Photomechanical Actuators with Photovoltaic Performance. Smart Mol. 2025, e70026. https://doi.org/10.1002/smo2.70026.
- 32.
Dai, J.; Xian, J.; Liu, K.; et al. Unconventional Metastable Cubic 2D LaMnO3 for Efficient Alkaline Seawater Oxygen Evolution. Chin. J. Catal. 2025, 74, 228–239.
- 33.
Ge, Y.; Yong, M.; Zeng, X.; et al. Biomass-Derived Materials for Advanced Vanadium Redox Flow Batteries. Mater. Futures 2025, 4, 042104.
- 34.
Wang, Y.; Li, P.; Liu, B.; et al. Solid Polymer Electrolytes: Ion Conduction Enhancement and Comprehensive Frontiers. Mater. Futures 2025, 4, 042103.
- 35.
Lee, J.G.; Hwang, J.; Hwang, H.J.; et al. A New Family of Perovskite Catalysts for Oxygen-Evolution Reaction in Alkaline Media: BaNiO3 and BaNi0.83O2.5. J. Am. Chem. Soc. 2016, 138, 3541–3547.
- 36.
Forslund, R.P.; Hardin, W.G.; Rong, X.; et al. Exceptional Electrocatalytic Oxygen Evolution via Tunable Charge Transfer Interactions in La0.5Sr1.5Ni1−xFexO4±δ Ruddlesden-Popper Oxides. Nat. Commun. 2018, 9, 3150.
- 37.
Tan, Y.; Jin, H.; Mao, S.S.; et al. Surface Hydrophobicity-Hydrophilicity Switching Induced Interface Heat and Water Transfer Enhancement for High-Efficiency Solar Steam Generation. Carbon Neutrality 2023, 2, 11.
- 38.
Wu, Z.; Xian, J.; Dai, J.; et al. Microwave-Pulse Synthesis of Tunable 2D Porous Nickel-Enriched LaMnxNi1−xO3 Solid Solution for Efficient Electrocatalytic Urea Oxidation. J. Mater. Chem. A 2024, 12, 7047–7057.
- 39.
Fan, L.; Rautama, E.L.; Lindén, J.; et al. Two Orders of Magnitude Enhancement in Oxygen Evolution Reactivity of La0.7Sr0.3Fe1−xNixO3−δ by Improving the Electrical Conductivity. Nano Energy 2022, 93, 106794.
- 40.
Enoch, C.M.; Ingavale, S.; Marbaniang, P.; et al. Molten Salt-Directed Synthesis of Strontium Manganese Perovskite Oxide: An Active Electrocatalyst for the Oxygen Reduction Reaction and Oxygen Evolution Reaction. J. Mater. Chem. A 2023, 11, 21780–21792.
- 41.
Wan, J.; Hu, R.; Li, J.; et al. A Universal Construction of Robust Interface between 2D Conductive Polymer and Cellulose for Textile Supercapacitor. Carbohydr. Polym. 2022, 284, 119230.
- 42.
Hong, W.T.; Stoerzinger, K.A.; Lee, Y.-L.; et al. Charge-Transfer-Energy-Dependent Oxygen Evolution Reaction Mechanisms for Perovskite Oxides. Energy Environ. Sci. 2017, 10, 2190–2200.
- 43.
Liu, X.; de Camargo Branco, D.; An, L.; et al. Ultrafast Laser Shock Straining in Chiral Chain 2D Materials: Mold Topology-Controlled Anisotropic Deformation. Nano Micro Lett. 2025, 18, 83.
- 44.
Yan, Y.; Zhao, C.; Hui, J.; et al. Molecular Nonchemically Amplified Resists Based on Spirobixanthene Backbone: Sulfoxime Oxime Esters versus Sulfonium Salts. Smart Mol. 2025, e70028. https://doi.org/10.1002/smo2.70028.
- 45.
Wexler, R.B.; Gautam, G.S.; Stechel, E.B.; et al. Factors Governing Oxygen Vacancy Formation in Oxide Perovskites. J. Am. Chem. Soc. 2021, 143, 13212–13227.
- 46.
Wan, J.; Zhang, G.; Jin, H.; et al. Microwave-Assisted Synthesis of Well-Defined Nitrogen Doping Configuration with High Centrality in Carbon to Identify the Active Sites for Electrochemical Hydrogen Peroxide Production. Carbon 2022, 191, 340–349.
- 47.
Liu, D.; Zhou, P.; Bai, H.; et al. Development of Perovskite Oxide-Based Electrocatalysts for Oxygen Evolution Reaction. Small 2021, 17, 2101605.
- 48.
Wan, J.; Huang, L.; Wu, J.; et al. Rapid Synthesis of Size-Tunable Transition Metal Carbide Nanodots Under Ambient Conditions. J. Mater. Chem. A 2019, 7, 14489–14495.
- 49.
Zhao, J.-W.; Li, Y.; Luan, D.; et al. Structural Evolution and Catalytic Mechanisms of Perovskite Oxides in Electrocatalysis. Sci. Adv. 2024, 10, eadq4696.
- 50.
Ruiying, F.; Lianchao, W.; Xutian, Y.; et al. Defects-Engineered Metal-Organic Frameworks for Supercapacitor Platform. Sustain. Eng. Novit. 2025, 1, 2.
- 51.
Li, W.; Jiang, K.; Li, Z.; et al. Origin of Improved Photoelectrochemical Water Splitting in Mixed Perovskite Oxides. Adv. Energy Mater. 2018, 8, 1801972.
- 52.
Li, S.-F.; Zhang, B.-Q.; Wang, Y.-Q.; et al. Enhancing Oxygen Evolution Reaction Performance of Ruddlesden–Popper Perovskite Oxide through Heteroatom Incorporation. Chem. Eng. J. 2024, 491, 151912.
- 53.
Yu, N.; Ma, Y.; Ren, J.-K.; et al. High Negative Voltage Activating Perovskite Oxide with Bi-Vacancy Synergistic Regulation for Water Oxidation. Chem. Eng. J. 2023, 478, 147415.
- 54.
Liu, W.; Kawano, K.; Kamiko, M.; et al. Effects of A-site Cations in Quadruple Perovskite Ruthenates on Oxygen Evolution Catalysis in Acidic Aqueous Solutions. Small 2022, 18, 2202439.
- 55.
Wang, L.; Yang, Z.; Bowden, M.E.; et al. Hole-Trapping-Induced Stabilization of Ni4+ in SrNiO3/LaFeO3 Superlattices. Adv. Mater. 2020, 32, 2005003.
- 56.
Nguyen, T.X.; Lee, C.-H.; Sun, J.-H.; et al. Synergistic Modulation of Electronic Structure in High Entropy Perovskite Oxide for Enhanced Bifunctional Oxygen Evolution/Reduction Reactions and Its Mechanistic Insights via In-Situ Analyses and Density Functional Theory Calculation. Chem. Eng. J. 2025, 511, 161731.
- 57.
Yan, X.; Qi, J.; Wang, H.; et al. Tuning Interlayer and Mixed Vanadium Valences of V2O5 via Organic and Inorganic Guests Co-Intercalation Enables Boosted Aqueous Zinc-Ion Storage. Carbon Neutralization 2025, 4, e70082.
- 58.
Hou, W.; Feng, P.; Guo, X.; et al. Catalytic Mechanism of Oxygen Vacancies in Perovskite Oxides for Lithium–Sulfur Batteries. Adv. Mater. 2022, 34, 2202222.
- 59.
Wu, C.; Sun, Y.; Wen, X.; et al. Adjusting Oxygen Vacancies in Perovskite LaCoO3 by Electrochemical Activation to Enhance the Hydrogen Evolution Reaction Activity in Alkaline Condition. J. Energy Chem. 2023, 76, 226–232.
- 60.
Zhu, K.; Wu, T.; Li, M.; et al. Perovskites Decorated with Oxygen Vacancies and Fe–Ni Alloy Nanoparticles as High-Efficiency Electrocatalysts for the Oxygen Evolution Reaction. J. Mater. Chem. A 2017, 5, 19836–19845.
- 61.
Cai, Z.; Kuru, Y.; Han, J.W.; et al. Surface Electronic Structure Transitions at High Temperature on Perovskite Oxides: The Case of Strained La0.8Sr0.2CoO3 Thin Films. J. Am. Chem. Soc. 2011, 133, 17696–17704.
- 62.
Lopes, P.P.; Chung, D.Y.; Rui, X.; et al. Dynamically Stable Active Sites from Surface Evolution of Perovskite Materials during the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2021, 143, 2741–2750.
- 63.
Feng, Y.; Wang, M.; Zhang, H.; et al. Engineering Electrocatalytic Structures Through Molten Salt-Mediated Mechanistic Control. EcoEnergy 2025, 3, e70022.
- 64.
Guo, H.; Yang, Y.; Yang, G.; et al. Ex Situ Reconstruction-Shaped Ir/CoO/Perovskite Heterojunction for Boosted Water Oxidation Reaction. ACS Catal. 2023, 13, 5007–5019.
- 65.
Kuanhong, C.; Guangshu, Y.; Mingxuan, L.; et al. Organoselenium Catalyzed Reaction: Sustainable Chemistry from Laboratory to Industry. Sustain. Eng. Novit. 2025, 1, 1.
- 66.
Du, T.; Zheng, S.; Wu, C.; et al. Zeolite-Based Advanced Battery Separators: Synergistic Innovations in Structure Performance Application. Carbon Neutralization 2025, 4, e70068.
- 67.
Hu, Y.; Wei, L.; Chen, H.; et al. Quantifying Dynamic Changes of Oxygen Nonstoichiometry and Formation of Surface Phases of SrCoOx Electrocatalysts by Operando Characterizations. ACS Nano 2025, 19, 13999–14009.
- 68.
Fabbri, E.; Nachtegaal, M.; Binninger, T.; et al. Dynamic Surface Self-Reconstruction is the Key of Highly Active Perovskite Nano-Electrocatalysts for Water Splitting. Nat. Mater. 2017, 16, 925–931.
- 69.
Wang, B.; Wu, X.; Jia, S.; et al. Ultrahigh Specific Surface Area Mesoporous Perovskite Oxide Nanosheets with Rare-Earth-Enhanced Lattice Oxygen Participation for Superior Water Oxidation. J. Mater. Sci. Technol. 2025, 227, 255–261.
- 70.
Boucly, A.; Artiglia, L.; Fabbri, E.; et al. Direct Evidence of Cobalt Oxyhydroxide Formation on a La0.2Sr0.8CoO3 Perovskite Water Splitting Catalyst. J. Mater. Chem. A 2022, 10, 2434–2444.
- 71.
Cao, L.; Petracic, O.; Wei, X.-K.; et al. Migration Kinetics of Surface Ions in Oxygen-Deficient Perovskite During Topotactic Transitions. Small 2021, 17, 2104356.
- 72.
Seo, M.H.; Park, H.W.; Lee, D.U.; et al. Design of Highly Active Perovskite Oxides for Oxygen Evolution Reaction by Combining Experimental and ab Initio Studies. ACS Catal. 2015, 5, 4337–4344.
- 73.
Hao, W.; Huang, G.; Ma, X.; et al. Economical Iron-Based Catalyst Electrode for Highly Stable Catalytic Industrial-Scale Overall Seawater Splitting. Carbon Neutrality 2024, 3, 37.
- 74.
Xu, Z.; Men, X.; Shan, Y.; et al. Electronic Reconfiguration Induced by Neighboring Exchange Interaction at Double Perovskite Oxide Interface for Highly Efficient Oxygen Evolution Reaction. Chem. Eng. J. 2022, 432, 134330.
- 75.
Yutong, F.; Jiao, D.; Mingjie, W.; et al. Unraveling Metastable Perovskite Oxides Insights from Structural Engineering to Synthesis Paradigms. Microstructures 2025, 5, 2025068.
- 76.
Han, R.; Shang, Y.-H.; Yang, Y. Corrigendum: Metallic Glass Roadmap. Mater. Futures 2025, 4, 049501.
- 77.
Pittkowski, R.; Divanis, S.; Klementová, M.; et al. Engendering Unprecedented Activation of Oxygen Evolution via Rational Pinning of Ni Oxidation State in Prototypical Perovskite: Close Juxtaposition of Synthetic Approach and Theoretical Conception. ACS Catal. 2021, 11, 985–997.
- 78.
Yongfei, Y.; Guangyu, F.; Miao, F.; et al. Leveraging Novel Microwave Techniques for Tailoring the Microstructure of Energy Storage Materials. Microstructures 2024, 4, 2024035.
- 79.
Shang, C.; Xiao, X.; Xu, Q. Coordination Chemistry in Modulating Electronic Structures of Perovskite-Type Oxide Nanocrystals for Oxygen Evolution Catalysis. Coord. Chem. Rev. 2023, 485, 215109.
- 80.
Wang, F.; Zhang, C.; Yang, H. Mixed B-site Ruddlesden-Popper Phase Sr2(RuxIr1−x)O4 Enables Enhanced Activity for Oxygen Evolution Reaction. J. Energy Chem. 2022, 70, 623–629.
- 81.
Zhu, F.; Hou, M.; Du, Z.; et al. Steam-Promoted Symmetry Optimizations of Perovskite Electrodes for Protonic Ceramic Cells. Energy Environ. Sci. 2024, 17, 7782–7791.
- 82.
Heo, Y.; Choi, S.; Bak, J.; et al. Symmetry-Broken Atom Configurations at Grain Boundaries and Oxygen Evolution Electrocatalysis in Perovskite Oxides. Adv. Energy Mater. 2018, 8, 1802481.
- 83.
Ye, Z.; Zhang, M.; Deng, J.; et al. An Emerging Liquid-Crystalline Conducting Polymer Thermoelectrics: Opportunities and Challenges. Nano-Micro Lett. 2025, 18, 82.
- 84.
Li, X.; Wang, X.; Ding, J.; et al. Engineering Active Surface Oxygen Sites of Cubic Perovskite Cobalt Oxides toward Catalytic Oxidation Reactions. ACS Catal. 2023, 13, 6338–6350.
- 85.
Wei, Y.; Hu, Y.; Da, P.; et al. Triggered Lattice-Oxygen Oxidation with Active-Site Generation and Self-Termination of Surface Reconstruction During Water Oxidation. Proc. Natl. Acad. Sci. USA 2023, 120, e2312224120.
- 86.
Dai, Y.; He, H.; Ouyang, M.; et al. In-Operando X-Ray Imaging for Sobering Examination of Aqueous Zinc Metal Batteries. Nano Micro Lett. 2025, 18, 85.
- 87.
Dai, J.; Wang, M.; Tian, H.; et al. Microwave Shock-Driven Thermal Engineering of Unconventional Cubic 2D LaMnO3 for Efficient Oxygen Evolution. J. Mater. Chem. A 2025, 13, 31002–31012.
- 88.
Wang, W.; Yang, Y.; Huan, D.; et al. An Excellent OER Electrocatalyst of Cubic SrCoO3−δ Prepared by a Simple F-Doping Strategy. J. Mater. Chem. A 2019, 7, 12538–12546.
- 89.
Bak, J.; Bae, H.B.; Chung, S.-Y. Atomic-Scale Perturbation of Oxygen Octahedra via Surface Ion Exchange in Perovskite Nickelates Boosts Water Oxidation. Nat. Commun. 2019, 10, 2713.
- 90.
Hong, Y.; Byeon, P.; Bak, J.; et al. Local-Electrostatics-Induced Oxygen Octahedral Distortion in Perovskite Oxides and Insight into the Structure of Ruddlesden–Popper Phases. Nat. Commun. 2021, 12, 5527.
- 91.
Du, S.B.; Wang, J.; Ma, F.T.; et al. Correlation of Composition, Crystal Structure, Reducibility and Catalytic Oxidation Activity on La-Mn-Ni-O System. Acta Phys. Chim. Sin. 1992, 8, 630–635.
- 92.
Qin, S.; Dai, J.; Tian, H.; et al. 3D Printing Driving Innovations in Extreme Low-Temperature Energy Storage. Virtual Phys. Prototyp. 2025, 20, e2459798.
- 93.
Bai, J.; Cheng, W.; Wang, T.; et al. Synergistic Regulation of Lithium Nucleation and Anion-Rich Solvation Structure via Silver Trifluoroacetate Additive for Stable Lithium Metal Anodes. Smart Mater. Devices 2025, 1, 202534.
- 94.
Etani, H.; Yamada, I.; Ohgushi, K.; et al. Suppression of Intersite Charge Transfer in Charge-Disproportionated Perovskite YCu3Fe4O12. J. Am. Chem. Soc. 2013, 135, 6100–6106.
- 95.
Song, J.; Zhu, S.C.; Ning, D.; et al. Defect Chemistry and Transport Properties of Perovskite-Type Oxides La1−xCaxFeO3−δ. J. Mater. Chem. A 2021, 9, 974–989.
- 96.
Olszewska, A.; Du, Z.; Świerczek, K.; et al. Novel ReBaCo1.5Mn0.5O5+δ (Re: La, Pr, Nd, Sm, Gd and Y) Perovskite Oxide: Influence of Manganese Doping on the Crystal Structure, Oxygen Nonstoichiometry, Thermal Expansion, Transport Properties, and Application as a Cathode Material in Solid Oxide Fuel Cells. J. Mater. Chem. A 2018, 6, 13271–13285.
- 97.
Jiang, H.; Xian, J.; Hu, R.; et al. Microwave Discharge for Rapid Introduction of Bimetallic-Synergistic Configuration to Conductive Catecholate Toward Long-Term Supercapacitor. Chem. Eng. J. 2023, 455, 140804.
- 98.
Liu, L.-B.; Tang, Y.-F.; Liu, S.; et al. Unraveling the Trade-Off Between Oxygen Vacancy Concentration and Ordering of Perovskite Oxides for Efficient Lattice Oxygen Evolution. Adv. Energy Mater. 2025, 15, 2402967.
- 99.
Feng, X.; Fan, Y.; Nomura, N.; et al. Graphene Promoted Oxygen Vacancies in Perovskite for Enhanced Thermoelectric Properties. Carbon 2017, 112, 169–176.
- 100.
Feng, Z.; Hong, W.T.; Fong, D.D.; et al. Catalytic Activity and Stability of Oxides: The Role of Near-Surface Atomic Structures and Compositions. Acc. Chem. Res. 2016, 49, 966–973.
- 101.
Wan, J.; Fang, G.; Mi, S.; et al. Metastable 2D Amorphous Nb2O5 for Aqueous Supercapacitor Energy Storage. Chem. Eng. J. 2024, 488, 150912.
- 102.
Yang, H.; Zhu, L.; Li, W.; et al. Lignocellulose-Mediated Gel Polymer Electrolytes Toward Next-Generation Energy Storage. Nano Micro Lett. 2025, 18, 84.
- 103.
Zuo, S.; Wang, C.; Xia, Z.; et al. Combined Exsolution and Electrodeposition Strategy for Enhancing Electrocatalytic Activity of Ti-Based Perovskite Oxides in Oxygen and Hydrogen Evolution Reactions. Adv. Sci. 2025, 12, 2410535.
- 104.
Zhang, S.; Wan, Y.; Xu, Z.; et al. Bismuth Doped La0.75Sr0.25Cr0.5Mn0.5O3−δ Perovskite as a Novel Redox-Stable Efficient Anode for Solid Oxide Fuel Cells. J. Mater. Chem. A 2020, 8, 11553–11563.
- 105.
Xu, X.; Pan, Y.; Zhong, Y.; et al. New Undisputed Evidence and Strategy for Enhanced Lattice-Oxygen Participation of Perovskite Electrocatalyst through Cation Deficiency Manipulation. Adv. Sci. 2022, 9, 2200530.
- 106.
Li, L.; Dong, Z.; Xia, T.; et al. A Series of Bifunctional ReBaCo2O5+δ Perovskite Catalysts Towards Intermediate-Temperature Oxygen Reduction Reaction and Oxygen Evolution Reaction. Chem. Eng. J. 2023, 468, 143762.
- 107.
Nguyen, L.T.; Cava, R.J. Hexagonal Perovskites as Quantum Materials. Chem. Rev. 2021, 121, 2935–2965.
- 108.
Zhao, C.; Zhang, X.; Yu, M.; et al. Cooperative Catalysis Toward Oxygen Reduction Reaction under Dual Coordination Environments on Intrinsic AMnO3-Type Perovskites via Regulating Stacking Configurations of Coordination Units. Adv. Mater. 2020, 32, 2006145.
- 109.
Yang, X.; Fernández–Carrión, A.J.; Geng, X.; et al. B-site Deficient Hexagonal Perovskites: Structural Stability, Ionic Order-Disorder and Electrical Properties. Prog. Solid State Chem. 2024, 74, 100459.
- 110.
Huang, J.; Tian, H.; Zhang, H.; et al. Two-Dimensional Materials for Enhanced Mid-Infrared Thermal Management. 2D Mater. 2025, 12, 032003.
- 111.
Fop, S.; Skakle, J.M.S.; McLaughlin, A.C.; et al. Oxide Ion Conductivity in the Hexagonal Perovskite Derivative Ba3MoNbO8.5. J. Am. Chem. Soc. 2016, 138, 16764–16769.
- 112.
Stitzer, K.E.; Darriet, J.; zur Loye, H.C. Advances in the Synthesis and Structural Description of 2H-Hexagonal Perovskite-Related Oxides. Curr. Opin. Solid State Mater. Sci. 2001, 5, 535–544.
- 113.
Tang, Z.; Zhang, X.; Huang, D.; et al. Critical Thickness and Its Role in the Spheroidization of Natural Flake Graphite. Carbon Neutralization 2026, 5, e70079.
- 114.
Huang, Y.; Xiao, H.; He, B.; et al. Probing Trace Pt Incorporated SrIrO3 Perovskite for Efficient and Stable Acidic Water Oxidation. J. Energy Chem. 2024, 99, 325–334.
- 115.
Wachi, K.; Makizawa, M.; Aihara, T.; et al. Oxygen Defect Engineering of Hexagonal Perovskite Oxides to Boost Catalytic Performance for Aerobic Oxidation of Sulfides to Sulfones. Adv. Funct. Mater. 2025, 35, 2425452.
- 116.
Fan, M.; Tian, H.; Wu, Z.; et al. Microwave Shock Synthesis of Porous 2D Non-Layered Transition Metal Carbides for Efficient Hydrogen Evolution. SusMat 2025, 5, e252.
- 117.
Zhou, B.W.; Zhang, J.; Ye, X.B.; et al. Octahedral Distortion and Displacement-Type Ferroelectricity with Switchable Photovoltaic Effect in a 3d3-Electron Perovskite System. Phys. Rev. Lett. 2023, 130, 146101.
- 118.
Christy, M.; Choi, S.; Kwon, J.; et al. The Perfect Imperfections of Perovskite Oxide Catalysts in the Aspect of Defect Equilibria. Small Sci. 2025, 5, 2400386.
- 119.
Cohen, R.E. Origin of Ferroelectricity in Perovskite Oxides. Nature 1992, 358, 136–138.
- 120.
Cohen, N.; Diéguez, O. Supertetragonal Phases of Perovskite Oxides: Insights from Electronic Structure Calculations. Isr. J. Chem. 2020, 60, 833–841.
- 121.
Xu, Z.; Qin, W.; Nisar, M.; et al. Phonon Scattering Engineering via Yb Doping in SnSe2 for Substantially Lowered Thermal Conductivity and Enhanced Thermoelectric Performance. Carbon Neutralization 2026, 5, e70083.
- 122.
Martin, A.; Khansur, N.H.; Urushihara, D.; et al. Effect of Li on the Intrinsic and Extrinsic Contributions of the Piezoelectric Response in Lix(Na0.5K0.5)1−xNbO3 Piezoelectric Ceramics Across the Polymorphic Phase Boundary. Acta Mater. 2024, 266, 119691.
- 123.
Martirosyan, M.; Passuti, S.; Masset, G.; et al. Nanoscale Characterization of Atomic Positions in Orthorhombic Perovskite Thin Films. Small 2025, 21, e02538.
- 124.
Roh, C.J.; Jung, M.-C.; Kim, J.R.; et al. Polar Metal Phase Induced by Oxygen Octahedral Network Relaxation in Oxide Thin Films. Small 2020, 16, 2003055.
- 125.
Ding, X.; Yang, B.; Leng, H.; et al. Crystal Symmetry Engineering in Epitaxial Perovskite Superlattices. Adv. Funct. Mater. 2021, 31, 2106466.
- 126.
Wang, G.J.; Xu, T.; Wen, S.; et al. Structure-Dependent Electrocatalytic Activity of La1−xSrxMnO3 for Oxygen Reduction Reaction. Sci. China Chem. 2015, 58, 871–878.
- 127.
Xu, R.; Zhu, Z.; Zhang, H.; et al. Synergistic Material–Structure Engineering for Mid-Infrared Thermal Management in Textiles. Small 2025, 21, e09257.
- 128.
Fu, J.; Zuo, R. Structural Evidence for the Polymorphic Phase Boundary in (Na,K)NbO3 Based Perovskites Close to the Rhombohedral-Tetragonal Phase Coexistence Zone. Acta Mater. 2020, 195, 571–578.
- 129.
Zhou, J.S.; Goodenough, J.B. Universal Octahedral-Site Distortion in Orthorhombic Perovskite Oxides. Phys. Rev. Lett. 2005, 94, 065501.
- 130.
Tahini, H.A.; Tan, X.; Schwingenschlögl, U.; et al. Formation and Migration of Oxygen Vacancies in SrCoO3 and Their Effect on Oxygen Evolution Reactions. ACS Catal. 2016, 6, 5565–5570.
- 131.
Wang, Q.; Gu, Y.; Zhu, W.; et al. Noble-Metal-Assisted Fast Interfacial Oxygen Migration with Topotactic Phase Transition in Perovskite Oxides. Adv. Funct. Mater. 2021, 31, 2106765.
- 132.
Li, Z.; Mao, X.; Feng, D.; et al. Prediction of Perovskite Oxygen Vacancies for Oxygen Electrocatalysis at Different Temperatures. Nat. Commun. 2024, 15, 9318.
- 133.
Yang, Y.; Lu, J.; Zhang, X.; et al. Symmetry-Induced Modulation of Proton Conductivity in Y-Doped Ba(Zr,Ce)O3: Insights from Raman Spectroscopy. J. Mater. Chem. A 2024, 12, 12599–12608.
- 134.
Huang, J.; Tian, H.; Zhang, H.; et al. Engineering Materials and Structural Paradigms for Mid-Infrared Thermal Management. Mater. Today Energy 2025, 52, 101944.
- 135.
Prosandeev, S.; Wang, D.; Ren, W.; et al. Novel Nanoscale Twinned Phases in Perovskite Oxides. Adv. Funct. Mater. 2013, 23, 234–240.
- 136.
Chen, L.; Yuan, Z.-Y. Electrocatalytic Alcohol and Aldehyde Oxidation: Advances in Catalysts and Reaction Mechanisms for Sustainable Chemical Synthesis. Smart Mater. Devices 2025, 2, 202531.
- 137.
Surta, T.W.; Whittle, T.A.; Wright, M.A.; et al. One Site, Two Cations, Three Environments: S2 and s0 Electronic Configurations Generate Pb-Free Relaxor Behavior in a Perovskite Oxide. J. Am. Chem. Soc. 2021, 143, 1386–1398.
- 138.
Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018.
- 139.
Hu, R.; Jiang, H.; Xian, J.; et al. Microwave-Pulse Sugar-Blowing Assisted Synthesis of 2D Transition Metal Carbides for Sustainable Hydrogen Evolution. Appl. Catal. B: Environ. 2022, 317, 121728.
- 140.
Li, W.; Chen, L.; Wang, D.; et al. Defect Engineering via Ag and Na Co-Doping in Wide-Bandgap CIGS: From Interfacial Suppression to Bulk Enhancement. Mater. Futures 2025, 4, 045105.
- 141.
Cheng, Y.; Wang, Y.; Shi, Z.; et al. Recent Progress in Advanced Design of Iridium-Based and Ruthenium-Based Perovskite Catalysts for Acidic Oxygen Evolution Reaction. EcoEnergy 2025, 3, 131–155.
- 142.
Zhou, H.; Zou, W.; Gu, S.; et al. Multimodal Intelligence in Chemical Discovery: Integrating Interpretable ML, Autonomous Robotics, and Edge Computing. Sustain. Eng. Novit. 2025, 1, 4.
- 143.
Li, J.; Du, Y.; Wang, K. Exceptionally Durable CO2 Photoreduction Mediated by Defect-Engineered CaIn2S4 Nanoflowers. EcoEnergy 2025, 3, e70020.
- 144.
Li, H.; Chen, Y.; Seow, J.Z.Y.; et al. Surface Reconstruction of Perovskites for Water Oxidation: The Role of Initial Oxides’ Bulk Chemistry. Small Sci. 2022, 2, 2100048.
- 145.
Jung, J.-I.; Park, S.; Kim, M.-G.; et al. Tunable Internal and Surface Structures of the Bifunctional Oxygen Perovskite Catalysts. Adv. Energy Mater. 2015, 5, 1501560.
- 146.
Riva, M.; Kubicek, M.; Hao, X.; et al. Influence of Surface Atomic Structure Demonstrated on Oxygen Incorporation Mechanism at a Model Perovskite Oxide. Nat. Commun. 2018, 9, 3710.
- 147.
Fang, G.; Ma, X.; Hu, R.; et al. Horizontally Oriented 2D Skin Structures on Fiber Interface for Long-Life Flexible Energy Storage Devices. Chem. Eng. J. 2025, 509, 161557.
- 148.
Wu, Z.; Fan, M.; Jiang, H.; et al. Harnessing the Unconventional Cubic Phase in 2D LaNiO3 Perovskite for Highly Efficient Urea Oxidation. Angew. Chem. Int. Ed. 2025, 64, e202413932.
- 149.
Xu, X.; Su, C.; Zhou, W.; et al. Co-Doping Strategy for Developing Perovskite Oxides as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Adv. Sci. 2016, 3, 1500187.
- 150.
Yu, Z.-Y.; Duan, Y.; Kong, Y.; et al. General Synthesis of Tube-Like Nanostructured Perovskite Oxides with Tunable Transition Metal–Oxygen Covalency for Efficient Water Electrooxidation in Neutral Media. J. Am. Chem. Soc. 2022, 144, 13163–13173.
- 151.
Hu, R.; Wei, L.; Xian, J.; et al. Microwave Shock Process for Rapid Synthesis of 2D Porous La0.2Sr0.8CoO3 Perovskite as an Efficient Oxygen Evolution Reaction Catalyst. Acta Phys. Chim. Sin. 2023, 39, 2212025.
- 152.
Wygant, B.R.; Jarvis, K.A.; Chemelewski, W.D.; et al. Structural and Catalytic Effects of Iron- and Scandium-Doping on a Strontium Cobalt Oxide Electrocatalyst for Water Oxidation. ACS Catal. 2016, 6, 1122–1133.
- 153.
Kim, U.; Lee, S.; Koo, D.; et al. Crystal Facet and Electronic Structure Modulation of Perovskite Oxides for Water Oxidation. ACS Energy Lett. 2023, 8, 1575–1583.
- 154.
Xu, X.; Chen, Y.; Zhou, W.; et al. Earth-Abundant Silicon for Facilitating Water Oxidation over Iron-Based Perovskite Electrocatalyst. Adv. Mater. Interfaces 2018, 5, 1701693.
- 155.
Ji, X.; Yang, F.; Du, Y.; et al. Highly Active and Durable La0.6Ca0.4(CrMnFeCo2Ni)O3 High Entropy Perovskite Oxide as Electrocatalyst for Oxygen Evolution Reaction in Alkaline Media. J. Mater. Sci. Technol. 2024, 168, 71–78.
- 156.
Kante, M.V.; Weber, M.L.; Ni, S.; et al. A High-Entropy Oxide as High-Activity Electrocatalyst for Water Oxidation. ACS Nano 2023, 17, 5329–5339.
- 157.
Hua, B.; Li, M.; Zhang, Y.-Q.; et al. All-In-One Perovskite Catalyst: Smart Controls of Architecture and Composition Toward Enhanced Oxygen/Hydrogen Evolution Reactions. Adv. Energy Mater. 2017, 7, 1700666.
- 158.
Li, S.; Xia, T.; Dou, Y.; et al. Phosphatizing Engineering of Perovskite Oxide Nanofibers for Hydrogen Evolution Reaction to Achieve Extraordinary Electrocatalytic Performance. Adv. Funct. Mater. 2022, 32, 2112164.
- 159.
Hua, B.; Li, M.; Pang, W.; et al. Activating p-Blocking Centers in Perovskite for Efficient Water Splitting. Chem 2018, 4, 2902–2916.
- 160.
Dai, J.; Zhu, Y.; Tahini, H.A.; et al. Single-Phase Perovskite Oxide with Super-Exchange Induced Atomic-Scale Synergistic Active Centers Enables Ultrafast Hydrogen Evolution. Nat. Commun. 2020, 11, 5657.
- 161.
Xu, X.; Pan, Y.; Zhong, Y.; et al. From Scheelite BaMoO4 to Perovskite BaMoO3: Enhanced Electrocatalysis Toward the Hydrogen Evolution in Alkaline Media. Compos. Part B Eng. 2020, 198, 108214.
- 162.
Zhang, L.; Jang, H.; Li, Z.; et al. SrIrO3 Modified with Laminar Sr2IrO4 as a Robust Bifunctional Electrocatalyst for Overall Water Splitting in Acidic Media. Chem. Eng. J. 2021, 419, 129604.
- 163.
Lu, Y.; Zhang, H.; Wang, Y.; et al. Solar-Driven Interfacial Evaporation Accelerated Electrocatalytic Water Splitting on 2D Perovskite Oxide/MXene Heterostructure. Adv. Funct. Mater. 2023, 33, 2215061.
- 164.
Wang, Y.; Lu, Q.; Ge, X.; et al. Molecular-Level Proton Acceptor Boosts Oxygen Evolution Catalysis to Enable Efficient Industrial-Scale Water Splitting. Green Energy Environ. 2024, 9, 344–355.
- 165.
Liu, Q.; Shen, F.; Song, G.; et al. Tailoring Ion Ordering in Perovskite Oxide for High-Temperature Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2023, 62, e202307057.
- 166.
Zhao, J.-W.; Yue, K.; Zhang, H.; et al. The Formation of Unsaturated IrOx in SrIrO3 by Cobalt-Doping for Acidic Oxygen Evolution Reaction. Nat. Commun. 2024, 15, 2928.
- 167.
Xian, J.; Jiang, H.; Wu, Z.; et al. Microwave Shock Motivating the Sr Substitution of 2D Porous GdFeO3 Perovskite for Highly Active Oxygen Evolution. J. Energy Chem. 2024, 88, 232–241.
- 168.
Retuerto, M.; Pascual, L.; Calle-Vallejo, F.; et al. Na-Doped Ruthenium Perovskite Electrocatalysts with Improved Oxygen Evolution Activity and Durability in Acidic Media. Nat. Commun. 2019, 10, 2041.
- 169.
Li, Q.; Wu, J.B.; Wu, T.; et al. Phase Engineering of Atomically Thin Perovskite Oxide for Highly Active Oxygen Evolution. Adv. Funct. Mater. 2021, 31, 2102002.
- 170.
Kim, J.; Yin, X.; Tsao, K.-C.; et al. Ca2Mn2O5 as Oxygen-Deficient Perovskite Electrocatalyst for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 14646–14649.
- 171.
Wang, J.; Gao, Y.; Chen, D.; et al. Water Splitting with an Enhanced Bifunctional Double Perovskite. ACS Catal. 2018, 8, 364–371.
- 172.
Tang, L.; Chen, Z.; Zuo, F.; et al. Enhancing Perovskite Electrocatalysis through Synergistic Functionalization of B-site Cation for Efficient Water Splitting. Chem. Eng. J. 2020, 401, 126082.
- 173.
Cui, X.; Li, W.; Liu, Y.; et al. Ultrafast Nanomanufacturing via High-Temperature Shock of La0.6Sr0.4CoO3 Catalysts for Overall Water Splitting. J. Mater. Sci. Technol. 2024, 191, 1–7.
- 174.
Zhai, T.; Wang, H.; Beaudoin, S.R.; et al. Perovskite Catalysts for Pure-Water-Fed Anion-Exchange-Membrane Electrolyzer Anodes: Co-Design of Electrically Conductive Nanoparticle Cores and Active Surfaces. J. Am. Chem. Soc. 2025, 147, 15448–15458.
- 175.
You, M.; Gui, L.; Ma, X.; et al. Electronic Tuning of SrIrO3 Perovskite Nanosheets by Sulfur Incorporation to Induce Highly Efficient and Long-Lasting Oxygen Evolution in Acidic Media. Appl. Catal. B Environ. 2021, 298, 120562.
- 176.
You, M.; Xu, Y.; He, B.; et al. Realizing Robust and Efficient Acidic Oxygen Evolution by Electronic Modulation of 0D/2D CeO2 Quantum Dots Decorated SrIrO3 Nanosheets. Appl. Catal. B Environ. 2022, 315, 121579.
- 177.
Zhao, L.; Tao, Z.; You, M.; et al. Partial Exsolution Enables Superior Bifunctionality of Ir@SrIrO3 for Acidic Overall Water Splitting. Adv. Sci. 2024, 11, 2309750.
- 178.
Sun, N.; Lai, Z.; Ding, W.; et al. Alkali Metals Activated High Entropy Double Perovskites for Boosted Hydrogen Evolution Reaction. Adv. Sci. 2024, 11, 2406453.
- 179.
Zhao, B.; Zhang, L.; Zhen, D.; et al. A Tailored Double Perovskite Nanofiber Catalyst Enables Ultrafast Oxygen Evolution. Nat. Commun. 2017, 8, 14586.
- 180.
Yagi, S.; Yamada, I.; Tsukasaki, H.; et al. Covalency-Reinforced Oxygen Evolution Reaction Catalyst. Nat. Commun. 2015, 6, 8249.
- 181.
Gunkel, F.; Jin, L.; Mueller, D.N.; et al. Ordering and Phase Control in Epitaxial Double-Perovskite Catalysts for the Oxygen Evolution Reaction. ACS Catal. 2017, 7, 7029–7037.
- 182.
Füngerlings, A.; Wohlgemuth, M.; Antipin, D.; et al. Crystal-Facet-Dependent Surface Transformation Dictates the Oxygen Evolution Reaction Activity in Lanthanum Nickelate. Nat. Commun. 2023, 14, 8284.
- 183.
Fan, Y.; Ye, X.; Zhou, J.; et al. Combined In Situ X-Ray Spectroscopic and Theoretical Study on Trimetal Synergistic Enhancement of Water Oxidation. Adv. Energy Mater. 2025, 15, 2404599.
- 184.
Weber, M.L.; Lole, G.; Kormanyos, A.; et al. Atomistic Insights into Activation and Degradation of La0.6Sr0.4CoO3−δ Electrocatalysts under Oxygen Evolution Conditions. J. Am. Chem. Soc. 2022, 144, 17966–17979.
- 185.
Sun, J.; Du, L.; Sun, B.; et al. A Bifunctional Perovskite Oxide Catalyst: The Triggered Oxygen Reduction/Evolution Electrocatalysis by Moderated Mn-Ni Co-Doping. J. Energy Chem. 2021, 54, 217–224.
- 186.
Weng, Z.; Huang, H.; Li, X.; et al. Coordination Tailoring of Epitaxial Perovskite-Derived Iron Oxide Films for Efficient Water Oxidation Electrocatalysis. ACS Catal. 2023, 13, 2751–2760.
- 187.
Yang, Y.; Chen, Y.; Yan, Y.; et al. Metal-Oxygen Octahedra Regulation of Iridium-Based Perovskites for Efficient and Durable Acidic Water Oxidation. Adv. Funct. Mater. 2025, 35, 2506467.
- 188.
Zhu, Y.; Zhou, W.; Zhong, Y.; et al. A Perovskite Nanorod as Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1602122.
- 189.
Oh, N.K.; Seo, J.; Lee, S.; et al. Highly Efficient and Robust Noble-Metal Free Bifunctional Water Electrolysis Catalyst Achieved via Complementary Charge Transfer. Nat. Commun. 2021, 12, 4606.
- 190.
Hu, C.; Hong, J.; Huang, J.; et al. Surface Decoration Accelerates the Hydrogen Evolution Kinetics of a Perovskite Oxide in Alkaline Solution. Energy Environ. Sci. 2020, 13, 4249–4257.
- 191.
Wang, L.; Stoerzinger, K.A.; Chang, L.; et al. Tuning Bifunctional Oxygen Electrocatalysts by Changing the A-Site Rare-Earth Element in Perovskite Nickelates. Adv. Funct. Mater. 2018, 8, 1803712.
- 192.
Zhang, X.; Tang, C.; Yang, Y.; et al. Novel High-Entropy Air Electrodes Enhancing Electrochemical Performances of Reversible Protonic Ceramic Cells. Adv. Funct. Mater. 2025, 35, 2421083.
- 193.
Nguyen, T.X.; Liao, Y.-C.; Lin, C.-C.; et al. Advanced High Entropy Perovskite Oxide Electrocatalyst for Oxygen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 2101632.
- 194.
Zhou, L.; Feng, D.; Li, Z.; et al. High-Spin-State Engineering in High-Entropy Perovskite Oxides via Crystal Phase Modulation for Paired Electrochemical Nitrate Reduction and Sulfur Ion Oxidation. Adv. Funct. Mater. 2025, e14375. https://doi.org/10.1002/adfm.202514375
- 195.
Satpathy, S.; Popović, Z.S.; Vukajlović, F.R. Electronic Structure of the Perovskite Oxides: La1−xCaxMnO3. Phys. Rev. Lett. 1996, 76, 960–963.
- 196.
Zhang, Y.; Lu, H.; Li, X.; et al. Engineering the Oxygen Vacancies in Perovskite Oxides for Electricity Generation from Water Evaporation. Adv. Mater. 2025, 38, e14202.
- 197.
Fang, G.; Liu, K.; Fan, M.; et al. Unveiling the Electron Configuration-Dependent Oxygen Evolution Activity of 2D Porous Sr-Substituted LaFeO3 Perovskite through Microwave Shock. Carbon Neutralization 2023, 2, 709–720.
- 198.
Jiang, H.; Li, J.; Xiao, Z.; et al. The Rapid Production of Multiple Transition Metal Carbides via Microwave Combustion Under Ambient Conditions. Nanoscale 2020, 12, 16245–16252.
- 199.
Pan, Y.; Xu, X.; Zhong, Y.; et al. Direct Evidence of Boosted Oxygen Evolution Over Perovskite by Enhanced Lattice Oxygen Participation. Nat. Commun. 2020, 11, 2002.
- 200.
Koo, B.; Kim, K.; Kim, J.K.; et al. Sr Segregation in Perovskite Oxides: Why It Happens and How It Exists. Joule 2018, 2, 1476–1499.
- 201.
Ingavale, S.; Gopalakrishnan, M.; Enoch, C.M.; et al. Strategic Design and Insights into Lanthanum and Strontium Perovskite Oxides for Oxygen Reduction and Oxygen Evolution Reactions. Small 2024, 20, 2308443.
- 202.
Qin, Y.; Fang, F.; Xie, Z.; et al. La,Al-Codoped SrTiO3 as a Photocatalyst in Overall Water Splitting: Significant Surface Engineering Effects on Defect Engineering. ACS Catal. 2021, 11, 11429–11439.
- 203.
Kim, B.-J.; Abbott, D.F.; Cheng, X.; et al. Unraveling Thermodynamics, Stability, and Oxygen Evolution Activity of Strontium Ruthenium Perovskite Oxide. ACS Catal. 2017, 7, 3245–3256.
- 204.
Chen, C.F.; King, G.; Dickerson, R.M.; et al. Oxygen-Deficient BaTiO3−x Perovskite as an Efficient Bifunctional Oxygen Electrocatalyst. Nano Energy 2015, 13, 423–432.
- 205.
Zhu, Y.; Liu, D.; Jing, H.; et al. Oxygen Activation on Ba-Containing Perovskite Materials. Sci. Adv. 2022, 8, eabn4072.
- 206.
Porokhin, S.V.; Nikitina, V.A.; Aksyonov, D.A.; et al. Mixed-Cation Perovskite La0.6Ca0.4Fe0.7Ni0.3O2.9 as a Stable and Efficient Catalyst for the Oxygen Evolution Reaction. ACS Catal. 2021, 11, 8338–8348.
- 207.
Lee, J.; Kim, S.; Kim, Y.B.; et al. Multiple Variations in LaCoO3 by Ca Substitution Activate Oxygen Evolution Reaction. Chem. Eng. J. 2025, 520, 165615.
- 208.
Ma, X.; Wu, Z.; Tian, H.; et al. Durable Coaxial Fiber-Based Underwater Strain Sensor with Reversible Dry–Wet Transition. InfoMat 2025, 7, e70030.
- 209.
Xu, X.; Pan, Y.; Ge, L.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573.
- 210.
Lei, J.; Wang, Z.; Zhang, Y.; et al. Understanding and Resolving the Heterogeneous Degradation of Anion Exchange Membrane Water Electrolysis for Large-Scale Hydrogen Production. Carbon Neutrality 2024, 3, 25.
- 211.
Zhou, W.; Xu, F.; Tan, J.; et al. An Angstrom-Scale Protective Skin Grown In Situ on Perovskite Oxide to Enhance Stability in Water. Angew. Chem. Int. Ed. 2025, 64, e202417360.
- 212.
Yu, J.; Liu, Q.; Wang, S.; et al. Spin-State Tuning in PrFeO3−δ Perovskite for High-Temperature Oxygen Evolution Reaction. J. Am. Chem. Soc. 2025, 147, 33086–33096.
- 213.
Wang, W.; Xu, M.; Xu, X.; et al. Perovskite Oxide Based Electrodes for High-Performance Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 136–152.
- 214.
Yuan, R.-h.; He, Y.; He, W.; et al. Bifunctional Electrocatalytic Activity of La0.8Sr0.2MnO3-Based Perovskite with the A-Site Deficiency for Oxygen Reduction and Evolution Reactions in Alkaline Media. Appl. Energy 2019, 251, 113406.
- 215.
Qian, X.; He, J.; Mastronardo, E.; et al. Outstanding Properties and Performance of CaTi0.5Mn0.5O3–δ for Solar-Driven Thermochemical Hydrogen Production. Matter 2021, 4, 688–708.
- 216.
Zhao, Y.; Liu, T.; Shi, Q.; et al. Perovskite Oxides La0.4Sr0.6CoxMn1−xO3 (x = 0, 0.2, 0.4) as an Effective Electrocatalyst for Lithium—Air Batteries. Green Energy Environ. 2018, 3, 78–85.
- 217.
Sung, M.-C.; Lee, G.-H.; Kim, D.-W. Efficient Li2O2 Oxidation Kinetics of Perovskite-Type Lanthanum Chromium-Based Oxide by Promoter Interface Formation for Lithium-Oxygen Batteries. Energy Storage Mater. 2023, 60, 102829.

This work is licensed under a Creative Commons Attribution 4.0 International License.


