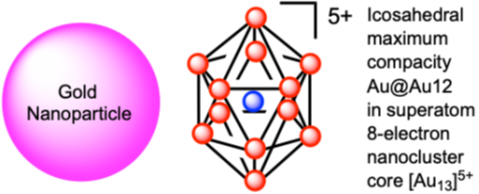
Gold nanoparticles (AuNPs), with size between 3 and 100 nm are well-known for their optical properties due to their plasmon band related to the resonance of the collective oscillation of their surface electron cloud matching the incident light wavelength. When the AuNPs are smaller than 2.3 nm, they are no longer plasmonic with fcc structures, but they fall in a quantum size regime of ultra-small AuNPs with specific structural, electronic, optical and catalytic properties resulting from molecular diagrams. They are atomically precise nanoclusters (APNCs), and their construction usually follows the Mackay maximum compacity with successive concentric shells of (10i2 + 2) atoms, meaning that for i = 1, the central Au atom is surrounded by 12 Au atoms Au@Au12 in many icosahedral Au13 clusters and the second layer (i = 1) contains 42 atoms with Au@Au12@Au42 in a Au55 cluster (Schmid’s cluster), etc. The icosahedron is the most frequently observed structure in Au APNCs, but the tetra-, hexa-, deca, icosa- and cubocta-hedron structures are also found, and all these symmetrical structures contribute to the cluster stability, as well as the surface Au-S staples. Since each Au atom contributes its single 6s electron to the non-bonding cluster valence electrons, an exceptional stability is obtained when the total number of non-bonding valence electrons contributing to the clusterification reaches one of the magic numbers 2, 8, 18, 34, 58, 92, 138, … corresponding to closed electron shells, when the compacity is high (quasi-spherical). For instance, the 2-electron Au3+ and 8-electron Au135+ cores are named superatom clusters. Many clusters, however, also deviate from this trend. The most common cluster series [Au25(SR)18]− composed of the 8-electron Au135+ core and 6[Au(I)2(SR)6]2− staples are superatom clusters. Although the size-focusing synthesis of the major groups of APNCs is that of thiolate clusters, whose accurate synthesis was astutely focused by the Jin group, the first 8-electron icosahedral phosphine Au13 cluster was predicted 50 years ago, then synthesized by the Mingos group in 1981, and in the 2010s new series of Au APNCs appeared with the alkynyl and carbene [both Bertrand-type and N-Heterocyclic Carbene (NHC)-type]) Au APNCs. All these APNC families present very promising electronic, photophysical and catalytic, (including electrocatalytic and photocatalytic) properties that are now exploited by researchers toward biomedicine and energy conversion processes, as well as those of small AuNPs whose catalytic properties were pioneered by Haruta.
- Open Access
- Review
Nano Gold: From Nanoparticles to Atomically Precise Nanoclusters †
Author Information
Received: 25 Sep 2025 | Revised: 14 Nov 2025 | Accepted: 18 Nov 2025 | Published: 04 Dec 2025
Abstract
Graphical Abstract
Keywords
gold nanoparticles | gold cluster | superatom | catalysis | atomically precise nanocluster
References
- 1.
Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346.
- 2.
Antonii, F. Panacea Aurea-Auro Potabile; Bibliopolio Frobeniano: Hamburg, Germany, 1618.
- 3.
Kahn, R.L. Serum Diagnosis for Syphilis. Colloid Chem. 1928, 2, 757.
- 4.
Faraday, M. Experimental Relations of Gold (and other Metals) to Light. Philos. Trans. 1857, 147, 145–181.
- 5.
Mie, G. Beiträge zur optik trüber medien, speziell kolloidaler metallösungen. Ann. Phys. 1908, 330, 377–445.
- 6.
Schmid, G. Large Clusters and Colloids. Chem. Rev. 1992, 92, 1709–1727.
- 7.
Jin, R.C.; Zeng, C.J.; Zhou, M.; Chen, Y.X. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413.
- 8.
Li, S.; Li, N.N.; Mak, T.C.W. Chemical Flexibility of Atomically Precise Metal Clusters. Chem. Rev. 2024, 124, 7262–7378.
- 9.
Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271.
- 10.
Chen, S.; Ingram, R.S.; Hostetler, M.J.; Pietron, J.J.; Murray, R.W.; Schaaff, T.G.; Whetten, R.L. Gold Nanoelectrodes of Varied Size: Transition to Molecule-Like Charging. Science 1998, 280, 2098–2101.
- 11.
Quinn, B.M.; Liljeroth, P.; Ruiz, V.; Laaksonen, T.; Kontturi, K. Electrochemical Resolution of 15 Oxidation States for Monolayer Protected Gold Nanoparticles. J. Am. Chem. Soc. 2003, 125, 6644–6645.
- 12.
Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; De Abajo, F.J.G. Modelling the optical response of gold nanoparticles. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805.
- 13.
Sun, Y.G.; Xia, Y.G. Shape-controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179.
- 14.
Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with Plasmonic Nanosensors. Nat. Mater. 2008, 7, 442–453.
- 15.
Boisselier, E.; Astruc, D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782.
- 16.
Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based Method for Rationally Assembling Nanoparticles into Macroscopic Materials. Nature 1996, 382, 607–609.
- 17.
Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120.
- 18.
Llevot, A. D. Astruc. Applications of Vectorized Gold Nanoparticles to the Diagnosis and Therapy of Cancer. Chem. Soc. Rev. 2012, 41, 242–257.
- 19.
Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Shin, H.S. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71.
- 20.
Kastner, S.; Dietel, A.K.; Seier, F.; Ghosh, S.; Weiß; D; Makarewicz, O.; Fritzsche, W. LSPR-based Biosensing Enables the Detection of Antimicrobial Resistance Genes. Small 2023, 19, e2207953.
- 21.
Gu, R.; Duan, Y.; Li, Y.; Luo, Z. Fiber-optic-based Biosensor as an Innovative Technology for Point-of-care Testing Detection of Foodborne Pathogenic Bacteria to Defend Food and Agricultural Product Safety. J. Agric. Food Chem. 2023, 71, 10982–10988.
- 22.
Qu, J.H.; Dillen, A.; Saeys, W.; Lammertyn, J.; Spasic, D. Advancements in SPR Biosensing Technology: An Overview of Recent Trends in Smart Layers Design, Multiplexing Concepts, Continuous Monitoring and in vivo Sensing. Anal. Chim. Acta. 2020, 1104, 10–27.
- 23.
Ravindran, N.; Kumar, S.; Yashini, M.; Rajeshwari, S.; Mamathi, C.A.; Nirmal, T.S.; Sunil, C.K. Recent advances in surface plasmon resonance (SPR) biosensors for food analysis: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1055–1077.
- 24.
Itoh, T.; Procházka, M.; Dong, Z.-C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Osaki, Y. Toward a new era of SERS and TERS at the nanometer scale: From fundamentals to innovative applications. Chem. Rev. 2023, 123, 1552–1634.
- 25.
Lin, H.C.; Lee, Y.; Lin, C.C.; Ho, Y.-L.; Xing, D.; Chen, M.-H.; Lin, B.-W.; Chen, L.-Y.; Chen, C.-W.; Delaunay, J.-J. Integration of on-chip perovskite nanocrystal laser and long-range surface plasmon polariton waveguide with etching-free process. Nanoscale 2022, 14, 10075–10081.
- 26.
Yang, L.; Wang, J.; Altreuter, J.; NJhaveri, A.; Wong, C.J.; Song, L.; Fu, J.; Taing, L.; Bodapati, S.; Sahu, A.; Tkheim, C.; Zhang, Y.; Zeng, Z.; Bai, G.; Tang, M.; Qiu, X.; Long, H.W.; Michor, F.; Liu, Y.; Liu, X.S. Tutorial: Integrative computational analysis of bulk RNA-sequencing data to characterize tumor immunity using RIMA. Nat. Protoc. 2023, 18, 2404–2414.
- 27.
Lu, F.; Astruc, D. Nanomaterials for Removal of Toxic Elements from Water. Coord. Chem. Rev. 2018, 356, 47–164.
- 28.
Nanda, B.P.; Rani, P.; Paul, P.; Aman; Ganti, S.S.; Bhatia, R. Recent Trends and Impact of Localized Surface Plasmon Resonance (LSPR) and Surface-enhanced Raman Spectroscopy (SERS) in Modern Analysis. J. Pharmaceut. Anal. 2024, 14, 100959.
- 29.
Saha, K.; Agasti, S.S.; Kim, C.; Li, X.N.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779.
- 30.
Turkevitch, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Process in the Synthesis of Colloidal Gold Discuss. Faraday Soc. 1951, 11, 55–75.
- 31.
Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nature: Phys. Sci. 1973, 241, 20–22.
- 32.
Yonezawa, T.; Kunitake, P. Practical Preparation of Anionic Mercapto Ligand-Stabilized Gold Nanoparticles and Their Immobilization. Colloids Surf. A Physicochem. Eng. Asp. 1999, 149, 193–199.
- 33.
Zhao, P.X.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665.
- 34.
Giersig, M.; Mulvaney, P. Preparation of Ordered Colloid Monolayers by Electrophoretic Deposition. Langmuir 1993, 9, 3408–3413.
- 35.
MBrust; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R.J. Synthesis of Thiol-Derivatized Gold Nanoparticles in a Two-phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802.
- 36.
BPrasad, L.V.; Stoeva, S.I.; Sorensen, C.M.; Klabunde, K.J. Digestive-Ripening Agents for Gold Nanoparticles: Alternatives to Thiols. Chem. Mater. 2003, 15, 935–942.
- 37.
Andres, R.P.; Bielefeld, J.D.; Henderson, J.I.; Janes, D.B.; Kolagunta, V.R.; Kubiak, C.P.; Mahoney, W.J.; Ogifsin, R.G. Self-Assembly of a Two-Dimensional Superlattice of Molecularly Linked Metal Clusters. Science 1996, 273, 1690–1693.
- 38.
Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C.J. Synthesis and Reactions of Functionalised Gold Nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 1655–1656.
- 39.
Hostetler, M.J.; Templeton, A.C.; Murray, R.W. Dynamics of Place-Exchange Reactions on Monolayer-Protected Gold Cluster Molecules. Langmuir 1999, 15, 3782–3789.
- 40.
Liu, M.Y.; Guo, R.-T.; Liu, C.; Cui, H.F.; Zhu, H.W.; Pan, W.G. Research progress in photocatalytic reduction of CO2 based on metal nanocluster materials. J. Mater. Chem. A 2024, 12, 32665–32688.
- 41.
Xia, Y.N.; Xiong, Y.J.; Skrabalak, S.E. Shape-controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics. Angew. Chem. Int. Ed. 2009, 48, 60–103.
- 42.
Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789.
- 43.
Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2013, 15, 1957–1962.
- 44.
Shiratori, K.; West, C.A.; Jia, Z.; Lee, S.A.; Cook, E.A.; Murphy, C.J.; Landes, C.F.; Link, S. Machine Learning to Adaptively Predict Gold Nanorod Sizes on Different Substrates. J. Phys. Chem. C 2025, 129, 5913–5920.
- 45.
Jin, R.; Qian, H.; Wu, Z.; Zhu, Y.; Zhu, M.; Mohanty, A.; Garg, N. Size Focusing: A Methodology for Synthesizing Atomically Precise Gold Nanoclusters, J. Phys. Chem. Lett. 2010, 1, 2903–2910.
- 46.
Cotton, F.A. Transition-metal Compounds Containing Clusters of Metal Atoms. Q. Rev. Chem. Soc. 1966, 20, 389–401.
- 47.
Astruc, D. Electron-Transfer Chain Catalysis in Organo-Transition-Metal Chemistry. Angew. Chem., Int. Ed. Engl. 1988, 27, 643–660.
- 48.
Alonso, E.; Astruc, D. Introduction of the Cluster Fragment Ru3(CO)11 at the Periphery of Phosphine Dendrimers Catalyzed by the Electron-Reservoir Complex [FeICp(C6Me6)]. J. Am. Chem. Soc. 2000, 122, 3222–3223.
- 49.
Goswani, N.; Yao, Q.; Chen, T.; Xie, J. Mechanistic Exploration and Controlled Synthesis of Precise Thiolate-gold Nanoclusters. Coord. Chem. Rev. 2016, 329, 1–15.
- 50.
Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-protected Gold Clusters Revisited: Bridging the Gap Between Gold(I)-thiolate Complexes and Thiolate-Protected Gold Nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270.
- 51.
Wu, Z.; MacDonald, M.A.; Chen, J.; Zhang, P. Jin. Kinetic Control and Thermodynamic Selection in the Synthesis of Atomically Precise Gold Nanoclusters. J. Am. Chem. Soc. 2011, 133, 9670–9673.
- 52.
Kang, X.; Chong, H.B.; Zhu, M.Z. Au25(SR)18: The Captain of the Great Nanocluster Ship. Nanoscale 2018, 10, 10758–10834.
- 53.
Zhu, M.; Lanni, E.; Garg, N.; Bier, M.E.; Jin, R. High-Yield Synthesis of Au25 Clusters, J. Am. Chem. Soc. 2008, 130, 1138.
- 54.
Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the Crystal Structure of a Thiol-Protected Au25 Cluster and Optical Properties. J. Am. Chem. Soc. 2008, 130, 5883–5885.
- 55.
Pan, P.Y.; Kang, X.; Zhu, M.Z. Preparation Methods of Metal Nanoclusters. Chem. Eur. J. 2025, 31, e202404528.
- 56.
Qian, H.; Zhu, Y.; Jin, R. Optical and Electrochemical Properties of Monodisperse Au38(SC2H4Ph)24 Nanoclusters. ACS Nano 2009, 3, 3795–3803.
- 57.
Qian, H.; Eckenhoff, W.T.; Zhu, Y.; Pintauer, T.; Jin, R. Total Structure Determination of Thiolate-Protected Au38 Nanoparticles. J. Am. Chem. Soc. 2010, 132, 8280–8281.
- 58.
Yan, J.Z.; Teo, B.K.; Zheng, N.F. Surface Chemistry of Atomically Precise Coinage-Metal Nanoclusters: From Structural Control to Surface Reactivity and Catalysis. Acc. Chem. Res. 2018, 51, 3084–3093.
- 59.
Matus, M.F.; Häkkinen, H. Understanding Ligand-protected Noble Metal Nanoclusters at Work. Nat. Rev. Mater. 2023, 8, 372–389.
- 60.
Yao, Q.F.; Zhu, M.S.Q.; Yang, Z.C.; Song, X.R.; Yuan, X.; Zhang, Z.P.; Hu, W.P.; Xie, J.P. Molecule-like synthesis of ligand-protected metal nanoclusters. Nat. Rev. Mater. 2024, 10, 89–108.
- 61.
Lyu, J.K.; Qian, J.; Yang, Z.C.; Xie, J.P. Synthesis planning for atomically precise metal nanoclusters. Nanoscale Horiz. 2025, 10, 2304–2339.
- 62.
Jin, S.; Wang, S.; Zhu, M.Z. Clusters and their alloys based on M13 units. Chem. Asian J. 2025, 14, 3222–3231.
- 63.
Wade, K. Structural Significance of number of skeletal bonding electron-pairs in carboranes, higher boranes and borane anions, and various transition-metal carbonyl cluster Compounds. J. Chem. Soc. Chem. Commun. 1971, 15, 792–793.
- 64.
Astruc, D. Organometallic Chemistry and Catalysis; Springer: Berlin, Germany, 2007; Chapter 2, pp. 47–76. https://doi.org/10.1007/978-3-540-46129-6.
- 65.
Chini, P. Large metal carbonyl clusters (LMCC). J. Organomet. Chem. 1980, 200, 37–61.
- 66.
Walter, M.; Akola, J.; Lopez-Acevedo, O.; Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Whetten, R.L.; Grönbeck, H.; Häkkinen, H. A unified view of ligand-protected gold clusters as superatom complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 9157–9162.
- 67.
I. Katakuse, T. Hichihara, Y. Fujita, T. Matsuo, T. Sakurai, H. Matsuda, Mass distributions of copper, silver and gold clusters and electronic shell structure. Intern. J. Mass Spectrom. Ion Process. 1985, 67, 229–236.
- 68.
Mingos, D.M.P. Molecular-orbital calculations on cluster compounds of gold. J. Chem. Soc. Dalton Trans. 1976, 13, 1163.
- 69.
Briant, C.E.; Theobald, B.R.C.; White, J.W.; Bell, L.K.; Mingos, D.M.P. Synthesis and X-ray crystal structure of the centred icosahedral gold cluster compound [Au13(PPhMe2)10Cl2] (PF6)3, the realization of a theoretical prediction. J. Chem. Soc. Chem. Commun. 1981, 5, 201–202.
- 70.
He, W.M.; Hu, H.; Cui, Y.J.; Li, J.; Si, Y.B.; Wang, S.B.; Zhao, Y.J.; Zhou, Z.; Ma, L.F.; Zang, S.-Q. Filling the gaps in icosahedral superatomic metal clusters. Nat. Sci. Rev. 2024, 11, 174.
- 71.
Heaven, W.; Dass, A.; White, P.S.; Holt, K.M.; Murray, R.W. Crystal structure of the gold nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18]. J. Am. Chem. Soc. 2008, 130, 1354–1355.
- 72.
Lavenn, C.; Albrieux, F.; Bergeret, G.; Chiriac, R.; Delichère, P.; Tuel, A. Functionalized gold magic clusters: Au25(SPhNH2)17. Nanoscale 2012, 4, 7334–7337.
- 73.
Akola, J.; Walter, M.; Whetten, R.; Häkkinen, H.; Grönbeck, H. On the Structure of Thiolate-Protected Au25. J. Am. Chem. Soc. 2008, 130, 3756–3757.
- 74.
Mackay, A.L. A dense non-crystallographic packing of equal spheres. Acta Crystallogr. 1962, 15, 916–918.
- 75.
Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 Å Resolution. Science 2007, 318, 430–433.
- 76.
Yao, Q.; Yuan, X.; Fung, V.; Yu, Y.; Leong, D.T.; Jiang, D.-E.; Xie, J. Understanding seed-mediated growth of gold nanoclusters at molecular level. Nat. Commun. 2017, 8, 927.
- 77.
Xiao, Q.; Chen, T.; Yuan, X.; Xie, J. Toward Total Synthesis of Thiolate-Protected Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 1338–1348.
- 78.
Kang, X.; Li, Y.; Zhu, M.Z.; Jin, R.C. Atomically precise alloy nanoclusters: Syntheses, structures, and properties. Chem. Soc. Rev. 2020, 49, 6443–6514.
- 79.
Maity, P.; Tsunoyama, H.; Yamauchi, M.; Xie, S.H.; Tsukuda, T. Organogold Clusters Protected by Phenylacetylene, J. Am. Chem. Soc. 2011, 133, 20123–20125.
- 80.
Maity, P.; Wakabayashi, T.; Ichikuni, N.; Tsunoyama, H.; Xie, S.; Yamauchi, M.; Tsukuda, T. Selective synthesis of organogold magic clusters Au54(C≡CPh)26. Chem. Commun. 2012, 48, 6085–6087.
- 81.
Maity, P.; Takano, S.; Yamazoe, S.; Wakabayashi, T.; Tsukuda, T. Binding Motif of Terminal Alkynes on Gold Clusters. J. Am. Chem. Soc. 2013, 135, 9450–9457.
- 82.
Wan, X.-K.; Guan, Z.-J.; Wang, Q.-M. Homoleptic Alkynyl-Protected Gold Nanoclusters: Au44(PhC≡C)28 and Au36(PhC≡C)24. Angew. Chem. Int. Ed. 2017, 56, 11494–11497.
- 83.
Lei, Z.; Li, J.-J.; Wan, X.K.; Wang, Q.M. Isolation and Total Structure Determination of an All-Alkynyl-Protected Gold Nanocluster Au144. Angew. Chem. Int. Ed. 2018, 57, 8639–8643.
- 84.
Lei, Z.; Wan, X.K.; Yuan, S.F.; Wang, J.Q.; Wang, Q.M. Alkynyl-protected gold and gold–silver nanoclusters. Dalton Trans. 2017, 46, 3427–3434.
- 85.
Wang, Y.; Su, H.; Xu, C.; Li, G.; Gell, L.; Lin, S.; Tang, Z.; Häkkinen, H.; Zheng, N. An Intermetallic Au24Ag20 Superatom Nanocluster Stabilized by Labile Ligands. J. Am. Chem. Soc. 2015, 137, 4324–4327.
- 86.
Wang, Y.; Wan, X.-K.; Ren, L.; Su, H.; Li, G.; Malola, S.; Lin, S.; Tang, Z.; Häkkinen, H.; Teo, B.K.; Wang, Q.-M.; Zheng, N. Atomically Precise Alkynyl-Protected Metal Nanoclusters as a Model Catalyst: Observation of Promoting Effect of Surface Ligands on Catalysis by Metal Nanoparticles. J. Am. Chem. Soc. 2016, 138, 3278–3281.
- 87.
Zeng, J.-L.; Guan, Z.-J.; Du, Y.; Nan, Z.-A.; Lin, Y.-M.; Wang, Q.-M. Chloride-Promoted Formation of a Bimetallic Nanocluster Au80Ag30 and the Total Structure Determination. J. Am. Chem. Soc. 2016, 138, 7848–7851.
- 88.
Lei, K.; Wan, X.-K.; Yuan, S.-F.; Guan, Z.-J. Alkynyl Approach toward the Protection of Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 2465–2474.
- 89.
MZhang, M.; Dong, X.-Y.; Wang, Y.-J.; Zang, S.-Q.; Mak, T.C.W. Recent progress in functional atom-precise coinage metal clusters protected by alkynyl ligands. Coord. Chem. Rev. 2022, 453, 214315.
- 90.
Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496.
- 91.
Zhou, Y.B.; Chen, W.Z. Synthesis and characterization of square-planar tetranuclear silver and gold clusters supported by a pyrazole-linked bis(N-heterocyclic carbene) ligand. Organometallics 2007, 26, 2742–2746.
- 92.
Vignolle, J.; Tilley, T.D. N-Heterocyclic carbene-stabilized gold nanoparticles and their assembly into 3D superlattices. Chem. Commun. 2009, 46, 7230–7232.
- 93.
Kiefer, C.; Bestgen, S.; Gamer, M.T.; Lebedkin, S.; Kapes, M.M.; Roesky, P.W. Alkynyl-functionalized gold NHC complexes and their coinage metal clusters. Dalton Trans. 2015, 44, 13662–13670.
- 94.
Scherbaum, F.; Groohmann, A.; Huber, B.; Krüger, C.; Schmidbauer, H. Aurophilicity as a Consequence of Relativistic Effects: The Hexakis(triphenylphosphaneaurio) methane Dication [(Ph3PAu)6C]2⊕. Angew. Chem. Int. Ed. 1988, 27, 1544–1546.
- 95.
Gabbaï, F.P.; Scheir, A.; Riede, J.; Scmidbauer, H. Synthesis of the Hexakis [(triphenylphosphane)gold(I)]methanium(2+) Cation from Trimethylsily ldiazomethane; Crystal Structure Determination of the Tetrafluoroborate Salt. Chem. Ber. 1997, 130, 111–114.
- 96.
Ube, H.; Zhang, Q.; Shinoya, M. A Carbon-Centered Hexagold(I) Cluster Supported by N-Heterocyclic Carbene Ligands. Organometallics 2018, 37, 2007–2009.
- 97.
Lei, Z.; Pei, N.-L.; Ube, H.; Shionoya, M. Reconstituting C-centered hexagold (I) clusters with N-heterocyclic carbene ligands. Bull. Chem. Soc. Jpn. 2021, 94, 1324–1330.
- 98.
Wei, J.; Halet, J.-F.; Kahlal, S.; Saillard, J.-Y.; Munoz-Castro, A. Toward the Formation of N-Heterocyclic-Carbene-Protected Gold Clusters of Various Nuclearities. A Comparison with Their Phosphine-Protected Analogues from Density Functional Theory Calculations. Inorg. Chem. 2020, 59, 15240–15249.
- 99.
Robilotto, T.J.; Bacsa, J.; Gray, T.G.; Sadighi, J.P. Synthesis of a Trigold Monocation: An Isolobal Analogue of [H3]+. Angew. Chem. Int. Ed. 2012, 51, 12077–12080.
- 100.
Jin, L.; Weinberger, D.S.; Melaimi, M.; Moore, C.E.; Rheingold, A.L.; Bertrand, G. Trinuclear Gold Clusters Supported by Cyclic (alkyl)(amino)carbene Ligands: Mimics for Gold Heterogeneous Catalysts. Angew. Chem., Int. Ed. 2014, 53, 9059–9063.
- 101.
Narouz, M.R.; Takano, S.; Lummis, P.A.; Levchenko, T.I.; Nazemi, A.; Kaappa, S.; Malola, S.; Yousefalizadeh, G.; Calhoun, L.A.; Stamplecoskie, K.G.; Häkkinen, H.; Tsukuda, T.; Robust, C.M. Highly Luminescent Au13 Superatoms Protected by N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2019, 141, 14997–15002.
- 102.
Shen, H.; Deng, G.; Kaappa, S.; Tan, T.; Han, Y.-Z.; Malola, S.; Lin, S.-C.; Teo, B.; Häkkinen, K.; Zheng, H.N. Highly Robust but Surface-Active: An N-Heterocyclic Carbene Stabilized Au25 Nanocluster. Angew. Chem. Int. Ed. 2019, 58, 17731–17735.
- 103.
Albright, E.L.; Levcheko, T.I.; Kulkarni, V.K.; Sullivan, A.I.; De Jesus, J.F.; Malola, S.; Takano, S.; Nambo, M.; Stamplekoskie, K.; Häkkinen, H.; Tsukuda, T.; Crudden, C.M. N-Heterocyclic Carbene-Stabilized Atomically Precise Metal Nanoclusters, J. Am. Chem. Soc. 2024, 146, 5759–5780.
- 104.
Shen, H.; Xiang, S.; Xu, Z.; Liu, C.; Li, X.; Sun, C.; Lin, S.; Teo, B.K.; Zheng, N. Superatomic Au13 clusters ligated by different N-heterocyclic carbenes and their ligand-dependent catalysis, photoluminescence, and proton sensitivity. Nano Res. 2020, 13, 1908–1911.
- 105.
Sun, J.; Tang, X.; Tang, J.; Zhang, Y.S.; Li, Z.; Cholumen; Guo, S.; Shen, H. Simple Approach toward N-Heterocyclic Carbene-Protected Gold Nanoclusters. Inorg. Chem. 2023, 62, 5088–5094.
- 106.
McPartlin, M.; Mason, R.; Malatesta, L. Novel cluster complexes of gold (0)-gold (I). J. Chem. Soc. D 1969, 7, 334.
- 107.
Adnan, R.H.; Madridejos, J.M.L.; Alotabi, A.S.; Metha, G.F.; Andersson, G.G. A Review of State of the Art in Phosphine Ligated Gold Clusters and Application in Catalysis. Adv. Sci. 2022, 9, 2105692.
- 108.
Mingos, D.M.P. Gold–A flexible friend in molecular chemistry. J. Chem. Soc. Dalton Trans. 1996, 5, 561–566.
- 109.
Mingos, D.M.P. Structural and bonding pattern in gold clusters. J. Chem. Soc. Dalton Trans. 2015, 44, 6680–6695.
- 110.
Ligare, M.R.; Johnson, G.E.; Laskin, J. Observing the real time formation of phosphine-ligated gold clusters by electrospray ionization mass spectrometry. Phys. Chem. Chem. Phys. 2017, 19, 17187.
- 111.
Pettibone, J.M.; Reardon, N.R. Nucleation products of ligated nanoclusters unaffected by temperature and reducing agent. Nanoscale 2012, 4, 5593.
- 112.
Kang, X.; Zhu, M.Z. Transformation of Atomically Precise Nanoclusters by Ligand-Exchange. Chem. Mater. 2019, 31, 24, 9939–9969.
- 113.
Anderson, D.P.; Alvino, J.F.; Gentleman, A.; Qahtani, H.A.; Thomsen, L.; Polson, M.I.J.; Metha, G.F.; Golovko, V.B.; Andersson, G.G. Chemically Synthesised Atomically Precise Gold Clusters Deposited and Activated on Titania. Phys. Chem. Chem. Phys. 2013, 15, 3917–3929.
- 114.
Schmid, G. Clusters and colloids-bridges between molecular and condensed materials. Mater. Chem. Phys. 1991, 29, 133–142.
- 115.
Schmid, G.; Liu, Y.-P.; Schumann, M.; Raschke, T.; Radehaus, C. Quasi One-Dimensional Arrangements of Au55(PPh3)12Cl6 Clusters and Their Electrical Properties at Room Temperature. Nano Lett. 2001, 1, 405–407.
- 116.
Jian, N.; Stapelfeldt, C.; Hu, K.-J.; Fröba, M.; Palmer, R.E. Hybrid atomic structure of the Schmid cluster Au55(PPh3)12Cl6 resolved by aberration-corrected STEM. Nanoscale 2015, 7, 885–888.
- 117.
Wen, F.; Englert, U.; Gutrath, B.; Simon, U. Electrochemical and Optical Properties of [Au9(PPh3)8](NO3)3. Eur. J. Inorg. Chem. 2008, 2008, 106–111.
- 118.
Gutrath, B.S.; Englert, U.; Wang, Y.; Simon, U. A Missing Link in Undecagold Cluster Chemistry: Single-Crystal X-ray Analysis of [Au11(PPh3)7Cl3]. Eur. J. Inorg. Chem. 2013, 2013, 2002–2006.
- 119.
Gutrath, B.S.; Schiefer, F.; Homberger, M.; Englert, U.; Şerb, M.-D.; Bettray, W.; Beljakov, I.; Meded, V.; Wenzel, W.; Simon, U. Molecular and Electronic Structure of the Cluster [Au8(PPh3)8](NO3)2. Eur. J. Inorg. Chem. 2016, 2016, 975–981.
- 120.
McKenzie, L.C.; Zaikova, T.O.; Hutchison, J.E. Structurally similar triphenylphosphine-stabilized undecagolds, Au11(PPh3)7Cl3 and [Au11(PPh3)8Cl2] Cl, exhibit distinct ligand exchange pathways with glutathione. J. Am. Chem. Soc. 2014, 136, 13426–13435.
- 121.
Xiao, F.-X.; Hung, S.F.; Miao, J.W.; Wang, H.Y.; Yang, H.B.; Liu, B. Metal-Cluster-Decorated TiO2 Nanotube Arrays: A Composite Heterostructure toward Versatile Photocatalytic and Photoelectrochemical Applications. Small 2015, 11, 554–567.
- 122.
Liu, C.; Abroshan, H.; Yan, C.; Li, G.; Haruta, M. One-Pot Synthesis of Au11(PPh2Py)7Br3 for the Highly Chemoselective Hydrogenation of Nitrobenzaldehyde. ACS Catal. 2016, 6, 92–99.
- 123.
Fernando, A.; Aikens, C.M. Ligand Exchange Mechanism on Thiolate Monolayer Protected Au25(SR)18Nanoclusters. J. Phys. Chem. C 2015, 119, 20179–20187.
- 124.
Pengo, P.; Bazzo, C.; Boccalon, M.; Pasquato, L. Differential Reactivity of the Inner and Outer Positions of Au25(SCH2CH2Ph)18 Dimeric Staples under Place Exchange Conditions. Chem. Commun. 2015, 51, 3204–3207.
- 125.
Kumara, C.; Aikens, C.M.; Dass, A. X-ray Crystal Structure and Theoretical Analysis of Au25–xAgx(SCH2CH2Ph)18–. Alloy J. Phys. Chem. Lett. 2014, 5, 461–466.
- 126.
Bose, P.; Roy, J.; Khokhar, V.; Mondal, B.; Natarajan, G.; Manna, S.; Yadav, V.; Yamijala, S.S.R.K.C.; Nyayban, A.; Nonappa; Pradeep, T. Interparticle Antigalvanic Reactions of Atomically Precise Silver Nanoclusters with Plasmonic Gold Nanoparticles: Interfacial Control of Atomic Exchange. Chem. Mater. 2024, 6, 7581–7594.
- 127.
Shibu, E.S.; Muhammed, M.A.H.; Tsukuda, T.; Pradeep, T. Ligand exchange of Au25SG18 leading to functionalized gold clusters: Spectroscopy, kinetics, and luminescenc. J. Phys. Chem. C 2008, 112, 12168–12176.
- 128.
Takano, S.; Hirai, H.; Muramatsu, S.; Tsukuda, T. Hydride-Doped Gold Superatom (Au9H)2+: Synthesis, Structure, and Transformation. J. Am. Chem. Soc. 2018, 140, 8380–8383.
- 129.
Liao, C.; Zhu, M.Z.; Jiang, D.-E.; Li, X.S. Manifestation of the interplay between spin-orbit and Jahn-Teller effects in Au25 superatom UV-Vis. fingerprint spectra. Chem. Sci. 2023, 14, 4666–4671.
- 130.
Pan, P.Y.; Zhang, L.D.; Wei, X.; Tian, Y.P.; Kang, X.; Zhang, Q.; Zhu, M.Z. Two-, and Three-Photon Excited Fluorescence of Atomically Precise Metal Nanoclusters. Angew. Chem. Int. Ed. 2022, 61, e202213016.
- 131.
Kang, X.; Zhu, M.Z. Tailoring the photoluminescence of atomically precise nanoclusters, Chem. Soc. Rev. 2019, 48, 2422–2457.
- 132.
Zhu, J.; Zhao, R.; Shen, H.L.; Zhu, C.; Zhou, M.; Kang, X.; Zhu, M.Z. Intramolecular Aggregation-Induced Surface Coupling of Metal Nanoclusters: Structure Elucidation and Photoluminescence Manipulation. Agregate 2025, 6, e720. https://doi.org/10.1002/agt2.720.
- 133.
Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975.
- 134.
Wang, S.; Meng, X.; Das, A.; Li, T.; Song, Y.; Cao, T.; Zhu, X.; Zhu, M.; Jin, R. A 200-Fold Quantum Yield Boost in the Photoluminescence of Silver-Doped AgxAu25–x Nanoclusters: The 13th Silver Atom Matters. Angew. Chem. Int. Ed. 2014, 53, 2376–2380.
- 135.
Bootharaju, M.S.; Kozlov, S.M.; Cao, Z.; Shkurenko, A.; El-Zohry, A.M.; Mohammed, O.F.; Eddaoudi, M.; Bakr, O.M.; Cavallo, L.; Basset, J.-M. Tailoring the Crystal Structure of Nanoclusters Unveiled High Photoluminescence via Ion Pairing. Chem. Mater. 2018, 30, 2719–2725.
- 136.
Ma, W.; Xu, L.G.; de Moura, A.; Wu, X.L.; Kuang, H.; Xu, C.L.; Kotov, N.A. Chiral inorganic nanostructures. Chem. Rev. 2017, 117, 8041–8093.
- 137.
Chen, Y.X.; Zeng, C.J.; Liu, C.; Kirschbaum, K.; Gayathri, C.; Gil, R.R.; Rosi, N.L.; Jin, R.C. Crystal Structure of Barrel-Shaped Chiral Au130(p-MBT)50 Nanocluster. J. Am. Chem. Soc. 2015, 137, 10076-100079.
- 138.
Dolamic, I.; Knoppe, S.; Dass, A.; Bürgi, T. First Enantioseparation and Circular Dichroism Spectra of Au38 Clusters Protected by Achiral Ligands. Nat. Commun. 2012, 3, 798.
- 139.
Zeng, C.; Chen, Y.; Kirschbaum, K.; Appavoo, K.; Sfeir, M.Y.; Jin, R. Structural Patterns at All Scales in a Nonmetallic Chiral Au133(SR)52. Sci. Adv. 2015, 1, e1500045.
- 140.
Shichibu, Y.; Ogawa, Y.; Sugiuchi, M.; Konishi, K. Chiroptical activity of Au13 clusters: Experimental and theoretical understanding of the origin of helical charge movements. Nanoscale Adv. 2021, 3, 1005–1011.
- 141.
Masatake, H.; Tetsuhiko, K.; Hiroshi, S.; Nobumasa, Y.; Haruta, M. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 16, 405–408.
- 142.
Okumura, M.; Kitagawa, Y.; Yamagcuhi, K.; Akita, T.; Tsubota, S.; Haruta, M. Direct Production of Hydrogen Peroxide from H2 and O2 over Highly Dispersed Au catalysts. Chem. Lett. 2003, 32, 822–823.
- 143.
Pina, D.; Falletta, E.; Rossi, M. Update on selective oxidation using gold. Chem. Soc. Rev. 2012, 41, 350.
- 144.
Chen, Y.; Liu, C.; Abroshan, H.; Li, Z.; Wang, J.; Li, G.; Haruta, M. One-Pot Synthesis of Au11(PPh2Py)7Br3 for the Highly Chemoselective Hydrogenation of Nitrobenzaldehyde. J. Catal. 2016, 340, 287.
- 145.
Bond, G.C.; Thompson, D.T. Catalysis by gold. Catal. Rev. Sci. Eng. 1999, 41, 319–388.
- 146.
Lu, F.; Ruiz, J.; Astruc, D. Nanoparticles as Recyclable Catalysts: The Fast-growing Frontier between Homogeneous and Heterogeneous Catalysts. Angew. Chem. Int. Ed. 2005, 44, 7852–7872.
- 147.
Astruc, D. Nanoparticles and Catalysis; Wiley-VCH: Weinhein, Germany, 2007.
- 148.
Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525.
- 149.
Yamamoto, K.; Imaoka, T.; Tanabe, M.; Gambe, T. New Horizon of Nanoparticle and Cluster Catalysis with Dendrimers, Chem. Rev. 2020, 120, 1397–1437.
- 150.
Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463.
- 151.
Jin, R.C.; Li, G.; Sharma, S.; Li, Y.W.; Du, X.S. Toward Active-Site Tailoring in Heterogeneous Catalysis by Atomically Precise Metal Nanoclusters with Crystallographic Structures. Chem Rev. 2021, 121, 567–648.
- 152.
Liu, Z.H.; Wu, Z.N.; Yao, Q.F.; Cao, Y.T.; Chai, Q.J.H.; Xie, J.P. Correlations between the fundamentals and applications of ultrasmall metal nanoclusters: Recent advances in catalysis and biomedical applications. Nano Today. 2021, 36, 101053.
- 153.
Du, Y.; Sheng, H.; Astruc, D.; Zhu, M.Z. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2020, 120, 526–622.
- 154.
Yang, D.; Pei, W.; Zhou, S.; Zhao, J.; Ding, W.; Zhu, Y. Controllable Conversion of CO2 on Non-Metallic Gold Clusters. Angew. Chem. Int. Ed. 2020, 59, 1919–1924.
- 155.
Nie, X.; Qian, H.; Ge, Q.; Xu, H.; Jin, R. CO Oxidation Catalyzed by Oxide-Supported Au25(SR)18 Nanoclusters and Identification of Perimeter Sites as Active Centers. ACS Nano 2012, 6, 6014–6022.
- 156.
Li, G.; Jin, R. Gold Nanocluster-Catalyzed Semihydrogenation: A Unique Activation Pathway for Terminal Alkynes. J. Am. Chem. Soc. 2014, 136, 11347–11354.
- 157.
Li, G.; Jiang, D.-E.; Liu, C.; Yu, C.; Jin, R. Oxide-Supported Atomically Precise Gold Nanocluster for Catalyzing Sonogashira Cross-Coupling. J. Catal. 2013, 306, 177–183.
- 158.
Kauffman, D.R.; Alfonso, D.; Matranga, C.; Qian, H.F.; Jin, R.C. Experimental and Computational Investigation of Au25 Clusters and CO2: A Unique Interaction and Enhanced Electrocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 10237–10243.
- 159.
Seong, H.; Efremo, V.; Park, G.; Kim, H.; Joo, J.S.; Lee, D. Atomically Precise Gold Nanoclusters as Model Catalysts for Identifying Active Sites for Electroreduction of CO2. Angew. Chem., Int. Ed. 2021, 60, 14563–14570.
- 160.
Narouz, M.R.; Osten, K.M.; Unsworth, P.J.; Man, R.W.Y.; Salorinne, K.; Takano, S.; Tomihara, R.; Kaappa, S.; Malola, S.; Dinh, C.T.; Padmos, J.D.; Ayoo, K.; Garrett, P.J.; Nambo, M.; Horton, J.H.; Sargent, E.H.; Häkkinen, H.; Tsukuda, T.; Crudden, C.M. N-heterocyclic carbene-functionalized magic-number gold nanoclusters. Nat. Chem. 2019, 11, 419–425.
- 161.
Zhao, J.; Ziarati, A.; Bûrgi, T. Tuning Atomically Precise Gold Nanoclusters for Selective Electroreduction of CO2. Angew. Chem. Int. Ed. 2025, 64, e202504320.
- 162.
Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216.
- 163.
Gellé, A.; Gin, T.; de la Garza, L.; Price, G.D.; Beistero, L.V.; Moores, A. Applications of Plasmon-Enhanced Nanocatalysis to Organic Transformations. Chem. Rev. 2020, 120, 986–1041.
- 164.
Kang, N.; Wei, X.; Shen, R.; Li, B.; Cal, E.G.; Moya, S.; Salmon, L.; Wang, C.; Coy, E.; Berlande, M.; Pozzo, J.-L.; Astruc, D. Fast Au-Ni@ZIF-8-Catalyzed Ammonia Borane Hydrolysis Boosted by Dramatic Volcano-Type Synergy and Plasmonic Acceleration. Appl. Catal. B 2023, 320, 121957.
- 165.
Zhao, Q.; Kang, N.; Moro, M.M.; Cal, E.G.; Moya, S.; Coy, E.; Salmon, L.; Liu, X.; Astruc, D. Sharp Volcano-Type Synergy and Visible-Light Acceleration in H2 Release upon B2(OH)4 Hydrolysis Catalyzed by Au-Rh@click-dendrimer Nanozymes. ACS Appl. Energy Mater. 2022, 5, 3834–3844.
- 166.
Wang, T.; Hamon, J.-R.; Wang, C. D. Astruc. Hydrogen production via nanocatalyzed ammonia borane hydrolysis: State of the art, recent progress and perspectives. Coord. Chem. Rev. 2025, 541, 216871.
- 167.
Liang, H.; Chen, Q.; Mo, Q.-L.; Wu, Y.; Xiao, F.-X. Atomically precise thiolate-protected goldnanoclusters: Current advances in solar-powered photoredox catalysis. J. Mater. Chem. A 2023, 11, 9401–9426.
- 168.
Kogo, A.; Sakai, N.; Tatsuma, T. Photocatalysis of Au25-Modified TiO2 under Visible and near Infrared Light. Electrochem. Commun. 2010, 12, 996–999.
- 169.
Sakai, N.; Tatsuma, T. Photovoltaic Properties of Glutathione-Protected Gold Clusters Adsorbed on TiO2 Electrodes. Adv. Mater. 2010, 22, 3185–3188.
- 170.
Li, T.; Zhang, R.R.; Fang, N.J.; Shi, Y.B.; Li, J.H.; He, C.S.; Chu, Y.H. Metal cluster-mediated photocatalysis: Synthesis; characterization; application. Nanoscale 2025, 17, 9834–9869.
- 171.
Zeng, Z.H.; Xiao, F.X.; Xu, Y.J. Atomically Precise Alloy Nanoclusters Steering Photocatalysis. Small 2025, 21, 2505282.
- 172.
Labande, A.; Ruiz, J.; Astruc, D. Supramolecular Gold Nanoparticles for the Redox Recognition of Oxoanions: Syntheses, Titrations, Stereoelectronic Efects, and Selectivity. J. Am. Chem. Soc. 2002, 124, 1782–1789.
- 173.
Muhammed, M.A.; Verma, P.K.; Pal, S.K.; Kumar, R.C.; Paul, S.; Omkumar, R.V.; Bright, T.B.P. Nir-Emitting Au23 from Au25: Characterization and Applications Including Biolabeling, Chem. Eur. J. 2009, 15, 10110–10120.
- 174.
Zheng, J.; Zhou, C.; Yu, M.X.; Liu, J.B. Different Sized Luminescent Gold Nanoparticles. Nanoscale 2012, 4, 4073–4083.
- 175.
Tan, X.; Jin, R. Ultrasmall Metal Nanoclusters for Bio-Related Applications. Nanomed. Nanobiotechnol. 2013, 5, 569–581.
- 176.
Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent Noble Metal Nanoclusters as an Emerging Optical Probe for Sensor Development. Chem. Asian J. 2013, 8, 858–871.
- 177.
Wang, S.; Zhu, X.Y.; Cao, T.T.; Zhu, M.Z. A simple model for understanding the fluorescence behavior of Au25 nanoclusters. Nanoscale 2014, 6, 5777–5781.
- 178.
Zhang, L.B.; Wang, E.K. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157.
- 179.
Zhang, X.-D.; Luo, Z.; Chen, J.; Shen, X.; Song, S.; Sun, Y.; Fan, S.; Fan, F.; Leong, D.T.; Xie, J. Ultrasmall Au10–12(SG)10–12 Nanomolecules for High Tumor Specificity and Cancer Radiotherapy Adv. Mater. 2014, 26, 4565–4568.
- 180.
He, L.Z.; Dong, T.T.; Jiang, D.E.; Zhang, Q.C. Recent progress in atomically precise metal nanoclusters: From bio-related properties to biological applications. Coord. Chem. Rev. 2025, 535, 216633.
- 181.
Sullivan, A.I.; Steele, E.A.; Takano, S.; Zeinizade, E.; Chen, J.; Malola, S.; Siddhant, K.; Häkkinen, H.; Stamplecoskie, K.G.; Tsukuda, T.; Zheng, G.; Crudden, C.M. Diving into Unknown Waters: Water-Soluble Clickable Au13Nanoclusters Protected with N-Heterocyclic Carbenes for Bio-Medical Applications. J. Am. Chem. Soc. 2025, 147, 4230–4238.
- 182.
Gallud, A.; Delaval, M.; Kinaret, P.A.S.; Marwa, V.S.; Fortino, V.; Ytterberg, J.; Zubarev, R.; Skoog, T.; Kere, J.; Correia, M.; Löchner, K.; Al-Ahmady, Z.; Kostarelos, K.; Ruiz, J.; Astruc, D.; Monopoli, M.; Moya, S.; Savolainen, K.; Alenius, H.; Tau, D.G.; Fadeel, B. Multi-Parametric Profiling of Engineered Nanomaterials: Unmasking the Surface Coating Effect. Adv. Sci. 2020, 7, 2002221.
- 183.
Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949.
- 184.
Li, H.; Kang, X.; Zhu, M.Z. Nanocluster-based aggregates: Assembled forms, driving forces, and structure-related properties. Coord. Chem. Rev. 2025, 539, 216738.
- 185.
Sethumadhavan, V. Nonappa, Atomically precise noble metal nanoclusters for engineering self-assembled two-dimensional materials. Dalton Trans. 2025, 54, 11770–11789.
- 186.
Nahan, E.N.; Aparna, R.K.; Chandrashekar, P.; Alam, N.; James, G.; Mandal, S. Ligand engineering for designing atomically precise metal nanoclusters and cluster-assemblies for potential applications. J. Chem. Sci. 2025, 137, 46.
- 187.
Yonesato, K.; Yanai, D.; Yamaguchi, K.; Suzuki, K. Recent Advances in Polyoxometalate-Metal Nanocluster Hybrids. Chem. Eur. J. 2025, 31, e202500877.
- 188.
Huang, J.H.; Cui, Y.; Wang, Z.Y.; Zang, S.-Q. Carborane Meets Metal Nanocluster: New Opportunities in Nanomaterials. Acc. Chem. Res. 2025, 58, 1249–1261.
- 189.
Barik, S.K.; Rao, M.S.K.; Jali, B.R.; Halet, J.-F.; Jena, H.S. Helical self-assemblies of molecular-like coinage metal nanoclusters assemblies and their emerging applications. Coord. Chem. Rev. 2025, 525, 216341.
- 190.
Zeng, L.L.; Zhou, M.; Jin, R.C. Evolution of Excited-State Behaviors of Gold Complexes, Nanoclusters and Nanoparticles. ChemPhysChem 2024, 25, e202300687.
- 191.
Zhu, H.G.; Wang, Z.P.; Yao, Q.F.; Zhang, Z.P.; Yao, Q.F.; Zhang, P.; Xie, J.P.; Yuan, X. Metal Nanoclusters for Cancer Imaging and Treatment. Adv. Funct. Mater. 2025, 35, e07924.
- 192.
Zheng, Y.K.; Wu, J.B.; Jiang, H.; Wang, X.M. Gold nanoclusters for theranostic applications. Coord. Chem. Rev. 2021, 431, 213689.
- 193.
Singh, J.; Alruwaili, N.K.; Aodah, A.; Almalki, W.H.; Almujri, S.S.; Alrobaian, M.; Rab, S.O.; Alanezi, A.A.; Haji, E.M.; Barkat, A. Gold nanoclusters in cancer drug delivery: Advances and emerging applications. J. Drug. Deliv. Sci. Technol. 2025, 105, 106594.
- 194.
Sarkar, S.; Das, J.; Debnath, B.; Ramzan, M.; Ashique, S.; Taghizadeh-Hesary, F. Nanobubbles: An emerging therapeutic paradigm for targeted cancer therapy. Bioinorg. Chem. 2025, 164, 108927.
- 195.
Sun, J.Q.; Cheng, W.V.; Liu, X.; Xiang, H.X.; Ruan, C.H.; Chen, F.Z.; Yao, C.H. Gold clusters: A promising NIR-II probe for bioimaging and biosensing. Coord. Chem. Rev. 2025, 533, 216544.
- 196.
Baghdasaryan, A.; Dai, H. Molecular Gold Nanoclusters for Advanced NIR-II Bioimaging and Therapy. Chem. Rev. 2025, 125, 5195–5227.
- 197.
Lu, T.Y.; Dong, J.X.; Li, Y.L.; Wang, J.J.; Su, M.; Fan, Y.J.; Shen, S.G.; Gao, Z.F. 0D Fluorescent Nanomaterials: Preparation, Properties, and its Antibacterial Applications. Adv. Nanobiomed. Res. 2025, 2500110. https://doi.org/10.1002/anbr.202500110.
- 198.
Gao, Y.X.; Kang, J.; Tian, H.Y.; Dong, A. Gold nanoparticle-based antibacterial materials and bioapplications. Soft Matter. 2025, 21, 6832–6851.
- 199.
Zhao, Y.; Zhou, H.M.; Qiao, L.L.; Ye, H.F.; Xu, J.M.; Antoine, R.; Deng, L.H.; Zhang, S.J.; Ye, H.F. Tumor-targeted ratiometric fluorescent gold nanoclusters for monitoring apoptosis and quantifying pH in vivo. Sens. Act. B Chem. 2025, 444, 138357.
- 200.
Guan, Z.J.; Li, J.J.; Hu, F.; Wang, Q.M. Structural Engineering toward Gold Nanocluster Catalysis. Angew. Chem. Int. Ed. 2022, 61, e20220972.
- 201.
Liu, X.; Cai, X.; Zhu, Y. Catalysis Synergism by Atomically Precise Bimetallic Nanoclusters Doped with Heteroatoms. Acc. Chem. Res. 2023, 56, 1528–1538.
- 202.
Wang, M.Y.; Chen, Y.; Tang, C. Recent Advances in Ligand Engineering for Gold Nanocluster Catalysis: Ligand Library, Ligand Effects and Strategies. Chem. Asian J. 2023, 18, e202300463.
- 203.
Yang, J.L.; Peng, Y.; Jia, Q.; Metal nanocluster-based hybrid nanomaterials: Fabrication and application. Coord. Chem. Rev. 2022, 456, 214391.
- 204.
Jing, W.T.; Shen, H.; Qin, R.X.; Wu, Q.Y.; Liu, K.L.; Zheng, N.F. Surface and Interface Coordination Chemistry Learned from Model Heterogeneous Metal Nanocatalysts: From Atomically Dispersed Catalysts to Atomically Precise Clusters. Chem. Rev. 2023, 123, 5948–6002.
- 205.
Zhao, J.T.; Ziarati, A.; Rosspeintner, A.; Bürgi, T. Anchoring of Metal Complexes on Au25 Nanocluster for Enhanced Photocoupled Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2024, 63, e202316649.
- 206.
Du, Y.X.; Fang, Y.; Wang, P.; Zhu, M.Z. Synergistic effects of atomically precise Au-based bimetallic nanocluster on energy-related small molecule catalysis. Chem. Sci. 2025, 16, 10642–10664.
- 207.
Du, Y.X.; Wang, P.; Fang, Y.; Zhu, M.Z. Asymmetric Charge Distribution in Atomically Precise Metal Nanoclusters for Boosted CO2 Reduction Catalysis. ChemSusChem. 2025, 18, e202402085.
- 208.
Xia, N.; Wang, W.; Zhuang, S.I.; Gu, W.M.; Li, J.; Deng, H.T.; Wang, Y.B.; Wu, Z.K. Electrochemical Carbon Dioxide Reduction Reaction or Hydrogen Evolution Reaction: Kernel and Type-Dependent Catalytic Activity of Staples in Metal Nanoclusters. Adv. Funct. Mater. 2025, 35, 2507721.
- 209.
Antoine, R.; Broyer, M.; Dugourd, P. Metal nanoclusters: From fundamental aspects to electronic properties and optical applications. Sci. Technol. Adv. Mater. 2023, 24, 2222546.
- 210.
Jameel, M.S.; Wahab, H.A.; Ahmad, W.; Mehrdel, B.; Alanezi, S.T.; Khaniabadi, P.M.; Deyab, M.A. Inorganic nanoparticles as smart carriers for docetaxel delivery in cancer therapy: Mechanisms, targeting strategies, and translational insights. J. Drug. Deliv. Sci. Technol. 2025, 114, 107464.
- 211.
Amiens, C.; Axet, M.R.; Igau, A.; Morales, E.M.; Philippot, K.; Romero, N.; Sutra, P. Metal complexes–metal nanoparticles hybrid systems, Coord. Chem. Rev. 2026, 547, 217053.
- 212.
Gauffre, F.; Coppel, Y.; Marty, J.D.; Mingotaud, C.; Kahn, M. When coordination chemistry meets soft matter: From hybrid nanoparticles to assemblies. Coord. Chem. Rev. 2025, 543, 216893.
- 213.
Hang, Y.J.; Wang, A.Y.; Wu, N.Q. Plasmonic silver and gold nanoparticles: Shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy, Chem. Soc. Rev. 2024, 53, 2932–2971.
- 214.
Liu, L.C.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079.
- 215.
Yang, H.C.; Duan, D.F.; Zhuang, Z.C.; Luo, Y.W.; Chen, J.; Xiong, Y.L.; Liu, X.W.; Wang, D.S. Understanding the Dynamic Evolution of Active Sites among Single Atoms, Clusters, and Nanoparticles. Adv. Mater. 2025, 37, 2415265.
- 216.
Wei, J.Y.; Kahlal, S.; Halet, J.-F.; Munoz-Castro, A.; Saillard, J.-Y. Ligand-Induced Cuboctahedral versus Icosahedral Core Isomerism within Eight-Electron Heterocyclic-Carbene-Protected Gold Nanoclusters. Inorg. Chem. 2022, 61, 8623–8628.
- 217.
Bühler, R.; Schütz, M.; Andriani, K.F.; Quiles, M.G.; de Mendonca, J.P.A.; Ocampo-Restrepo, V.K.; Stephan, J.; Ling, S.P.; Kahlal, S.; Saillard, J.-Y.; Gemel, C.; Da Silva, J.L.F.; Fischer, R.A. A living library concept to capture the dynamics and reactivity of mixed-metal clusters for catalysis. Nat. Chem. 2025, 17, 525–531.
- 218.
Shi, W.Q.; Zheng, L.L.; He, R.L.; Han, X.S.; Guan, Z.J.; Zhou, M.; Wang, Q.M. Near-unity NIR phosphorescent quantum yield from a room-temperature solvated metal nanocluster. Science 2024, 383, 326-330.
- 219.
Fang, F.; Zhu, N.S.Q.; Yao, Q.F.; Hu, W.P. Asymmetric catalysis promoted by hierarchical chirality of metal nanoclusters. Sci. China-Mater. 2025, 68, 3075–3092.
- 220.
Kim, S.; Jääskö, H.; Park, K.C.; Malola, S.; Häkkinen, H.; Park, S.S. Tuning the Electrical Properties through Metal-Ion-Mediated Assembly in Au25 Nanocluster-Based Frameworks. J. Am. Chem. Soc. 2025, 147, 30803–30808.

This work is licensed under a Creative Commons Attribution 4.0 International License.


