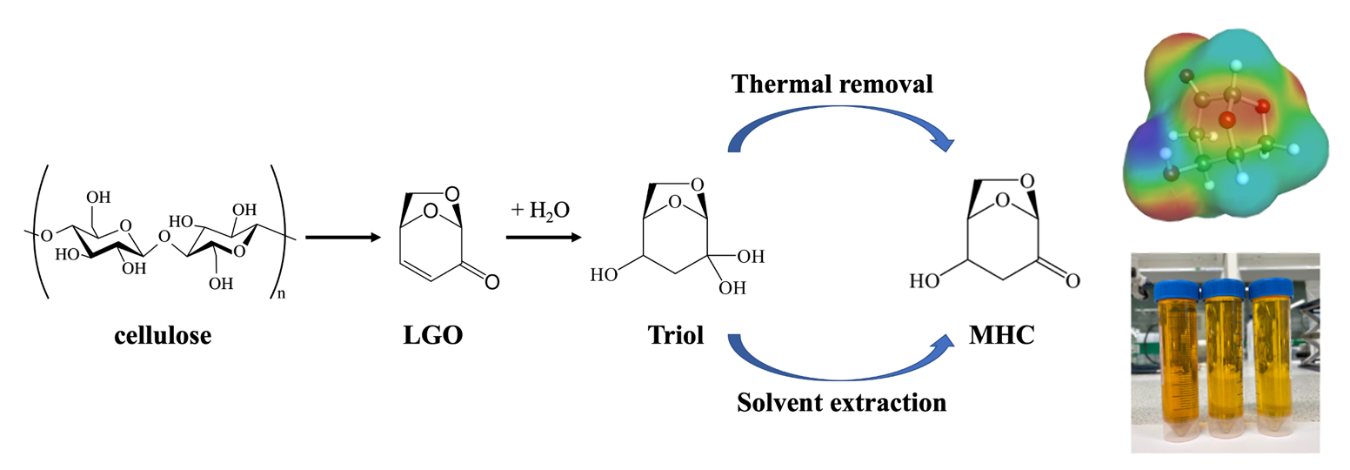
The importance of levoglucosenone (LGO) as a bio-derived platform molecule has been significantly elevated through its transformation into Cyrene™, a widely adopted green solvent. In this study, we investigate the interactions of LGO with water, a key component of biorefinery systems, revealing a new, efficient route to monohydroxycyrene (MHC). This transformation involves the slow, aqueous-based conversion of LGO to a triol intermediate, followed by selective dehydration to form MHC—a chiral molecule with dual functional groups and promising synthetic potential. MHC was synthesised in two simple and green steps without the need for catalysts or reagents, achieving an 88% yield and 98% purity under mild conditions. This environmentally benign approach aligns with the principles of green chemistry by eliminating the need for hazardous reagents and employing water as a sustainable solvent. The structure of MHC was confirmed using a combination of NMR, IR, UV-Vis, CHN, MS, and thermal analyses. Our results also highlight the role of temperature in influencing product formation, with lower temperatures (45–65 °C) enhancing yield, while higher temperatures (e.g., 95 °C) reduce conversion efficiency. MHC exhibits favourable physical and chemical properties, including polarity, solubility, and thermal stability, making it a promising candidate for future applications in green chemistry, pharmaceuticals, and materials science. The combined reactivity of the carbonyl and hydroxyl groups makes MHC a promissing platform molecule for synthesising polymers, pharmaceuticals, and advanced bio-based materials. Moreover, the mild reaction conditions and catalyst-free nature of the process contribute to reduced energy input and lower environmental impact. This work offers new insights into sustainable chemical pathways and provides a strong foundation for scaling up the production of novel biomass-derived building blocks.
- Open Access
- Article
A New Route to Monhydroxycyrene
- Majed Almuqhim 1,
- Con R. McElroy 1, 2, *,
- Jiajun Fan 1
Author Information
Received: 08 Apr 2025 | Revised: 08 Jul 2025 | Accepted: 23 Jul 2025 | Published: 04 Aug 2025
Abstract
Graphical Abstract
Keywords
References
- 1.Fankhauser, S.; Smith, S.M.; Allen, M.; et al. The meaning of net zero and how to get it right. Nat. Clim. Chang. 2022, 12, 15–21.
- 2.Christopher, S.; Vikram, M.; Bakli, C.; et al. Renewable energy potential towards attainment of net-zero energy buildings status–A critical review. J. Clean. Prod. 2023, 405, 136942.
- 3.Bonsu, N.O. Towards a circular and low-carbon economy: Insights from the transitioning to electric vehicles and net zero economy. J. Clean. Prod. 2020, 256, 120659.
- 4.Ritchie, H. Sector by Sector: Where Do Global Greenhouse Gas Emissions Come from? Available online: https://ourworldindata.org/ghg-emissions-by-sector (accessed on 11 June 2025).
- 5.Griffiths, S.; Sovacool, B.K.; Iskandarova, M.; et al. Bridging the gap between defossilization and decarbonization to achieve net-zero industry. Environ. Res. Lett. 2025, 20, 024063.
- 6.Sparks, J.; Scaldaferri, C.; Welfle, A.; et al. Carbon for Chemicals: How Can Biomass Contribute to the Defossilisation of the Chemicals Sector? University of Manchester: Manchester, UK, 2024.
- 7.Attard, T.M.; Clark, J.H.; McElroy, C.R. Recent developments in key biorefinery areas. Curr. Opin. Green Sustain. Chem. 2020, 21, 64–74.
- 8.Clark, J.H. Green biorefinery technologies based on waste biomass. Green Chem. 2019, 21, 1168–1170.
- 9.Awasthi, M.K.; Sindhu, R.; Sirohi, R.; et al. Agricultural waste biorefinery development towards circular bioeconomy. Renew. Sustain. Energy Rev. 2022, 158, 112122.
- 10.Van den Bosch, S.; Schutyser, W.; Vanholme, R.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763.
- 11.Mak, T.M.; Xiong, X.; Tsang, D.C.; et al. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297, 122497.
- 12.Govindasamy, G.; Jaya Balaji, P.K. Configuring municipal solid and liquid waste treatment plants into bio-refinery to achieve sustainable development goals. J. Mater. Cycles Waste Manag. 2025, 27, 2016–2031.
- 13.Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 2020, 300, 122755.
- 14.Farmer, T.J.; Mascal, M. Platform molecules. Introd. Chem. Biomass 2015, 89–155. https://doi.org/10.1002/9781118714478.ch4.
- 15.Tian, C.; Dorakhan, R.; Wicks, J.; et al. Progress and roadmap for electro-privileged transformations of bio-derived molecules. Nat. Catal. 2024, 7, 350–360.
- 16.Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; et al. Furfural production from biomass residues: Current technologies, challenges and future prospects. Biomass Bioenergy 2022, 161, 106458.
- 17.Rackemann, D.W.; Doherty, W.O. The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod. Biorefining 2011, 5, 198–214.
- 18.Prete, P.; Cespi, D.; Passarini, F.; et al. Glycidol syntheses and valorizations: Boosting the glycerol biorefinery. Curr. Opin. Green Sustain. Chem. 2022, 35, 100624.
- 19.Klement, T.; Büchs, J. Itaconic acid–A biotechnological process in change. Bioresour. Technol. 2013, 135, 422–431.
- 20.Allais, F. Total syntheses and production pathways of levoglucosenone, a highly valuable chiral chemical platform for the chemical industry. Curr. Opin. Green Sustain. Chem. 2023, 40, 100744.
- 21.Gomez, M.; Quincoces, J.; Kuhla, B.; et al. Synthesis of Push-Pull Derivatives of Levoglucosenone as Precursors of Annellated Pyranosides; Taylor Francis: Oxford, UK, 1999.
- 22.Tsai, Y.-h.; Etichetti, C.M.B.; Cicetti, S.; et al. Design, synthesis and evaluation of novel levoglucosenone derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2020, 30, 127247.
- 23.Camp, J.E.; Greatrex, B.W. Levoglucosenone: Bio-based platform for drug discovery. Front. Chem. 2022, 10, 902239.
- 24.Sherwood, J.; Constantinou, A.; Moity, L.; et al. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652.
- 25.Fan, J.; Budarin, V.; MacQuarrie, D.J.; et al. A new perspective in bio-refining: Levoglucosenone and cleaner lignin from waste biorefinery hydrolysis lignin by selective conversion of residual saccharides. Energy Environ. Sci. 2016, 9, 2571–2574.
- 26.Huang, X.; Kudo, S.; Asano, S.; et al. Improvement of levoglucosenone selectivity in liquid phase conversion of cellulose-derived anhydrosugar over solid acid catalysts. Fuel Process. Technol. 2021, 212, 106625.
- 27.Richardson, D.E.; Raverty, W.D. Predicted environmental effects from liquid emissions in the manufacture of levoglucosenone and Cyrene. Appita: Technol. Innov. Manuf. Environ. 2016, 69, 344–351.
- 28.Wilson, K.L.; Murray, J.; Jamieson, C.; et al. Cyrene as a bio-based solvent for HATU mediated amide coupling. Org. Biomol. Chem. 2018, 16, 2851–2854.
- 29.Camp, J.E. Bio-available solvent Cyrene: Synthesis, derivatization, and applications. ChemSusChem 2018, 11, 3048–3055.
- 30.Wilson, K.L.; Murray, J.; Jamieson, C.; et al. Cyrene as a bio-based solvent for the Suzuki–Miyaura cross-coupling. Synlett 2018, 29, 650–654.
- 31.ReSolute High Performing and Safe Solvent Derived from Celllulosic Feedstocks. Available online: https://www.cbe.europa.eu/projects/resolute (accessed on 17 June 2025).
- 32.Sheldon, R.A. Waste Valorization in a Sustainable Bio-Based Economy: The Road to Carbon Neutrality. Chem. A Eur. J. 2024, 30, e202402207.
- 33.Warne, C.M.; Fadlallah, S.; Whitwood, A.C.; et al. Levoglucosenone-derived synthesis of bio-based solvents and polyesters. Green Chem. Lett. Rev. 2023, 16, 2154573.
- 34.Stanfield, M.K.; Terry, R.S.; Smith, J.A.; et al. Levoglucosan and levoglucosenone as bio-based platforms for polymer synthesis. Polym. Chem. 2023, 14, 4949–4956.
- 35.Mouterde, L.M.; Allais, F.; Stewart, J.D. Enzymatic reduction of levoglucosenone by an alkene reductase (OYE 2.6): A sustainable metal-and dihydrogen-free access to the bio-based solvent Cyrene®. Green Chem. 2018, 20, 5528–5532.
- 36.Ma, X.; Liu, X.; Yates, P.; et al. Manipulating the enone moiety of levoglucosenone: 1, 3-Transposition reactions including ones leading to isolevoglucosenone. Tetrahedron 2018, 74, 5000–5011.
- 37.Sharipov, B.T.; Davydova, A.N.; Faizullina, L.K.; et al. Preparation of the diastereomerically pure 2S-hydroxy derivative of dihydrolevoglucosenone (cyrene). Mendeleev Commun. 2019, 29, 200–202.
- 38.Witczak, Z.J.; Kaplon, P.; Kolodziej, M. Thiosugars VI: A Simple Stereoselective Approach to (1→3)-3-S-Thiodisaccharides from Levoglucosenone; Springer: Berlin/Heidelberg, Germany, 2002.
- 39.Sharipov, B.T.; Davidova, A.N.; Ryabova, A.S.; et al. Synthesis and fungicidal activity of methylsulfanylmethyl ether derivatives of levoglucosenone. Chem. Heterocycl. Compd. 2019, 55, 31–37.
- 40.Witczak, Z.J.; Kaplon, P.; Kolodziej, M. Thiosugars VI: A Simple Stereoselective Approach to (1→3)-3-S-Thiodisaccharides from Levoglucosenone. In Timely Research Perspectives in Carbohydrate Chemistry; Springer: Berlin/Heidelberg, Germany, 2002; pp. 171–180.
- 41.Krishna, S.H.; Walker, T.W.; Dumesic, J.A.; et al. Kinetics of levoglucosenone isomerization. ChemSusChem 2017, 10, 129–138.
- 42.Diot-Néant, F.; Mouterde, L.; Couvreur, J.; et al. Green synthesis of 2-deoxy-D-ribonolactone from cellulose-derived levoglucosenone (LGO): A promising monomer for novel bio-based polyesters. Eur. Polym. J. 2021, 159, 110745.
- 43.Shafizadeh, F.; Furneaux, R.H.; Stevenson, T.T. Some reactions of levoglucosenone. Carbohydr. Res. 1979, 71, 169–191.
- 44.Mlostoń, G.; Urbaniak, K.; Palusiak, M.; et al. (3 + 2)-Cycloadditions of Levoglucosenone (LGO) with Fluorinated Nitrile Imines Derived from Trifluoroacetonitrile: An Experimental and Computational Study. Molecules 2023, 28, 7348.
- 45.Camp, J.; Greatrex, B. Levoglucosenone: Bio-based platform for drug discovery Front. Chem 2022, 10, 902239.
- 46.Plenert, A.C.; Mendez-Vega, E.; Sander, W. Micro-vs. macrosolvation in Reichardt’s dyes. J. Am. Chem. Soc. 2021, 143, 13156–13166.
- 47.Jessop, P.G.; Jessop, D.A.; Fu, D.; et al. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259.
How to Cite
Almuqhim, M.; McElroy, C. R.; Fan, J. A New Route to Monhydroxycyrene. Renewable Chemistry 2025, 1 (1), 3. https://doi.org/10.53941/rc.2025.100003.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


