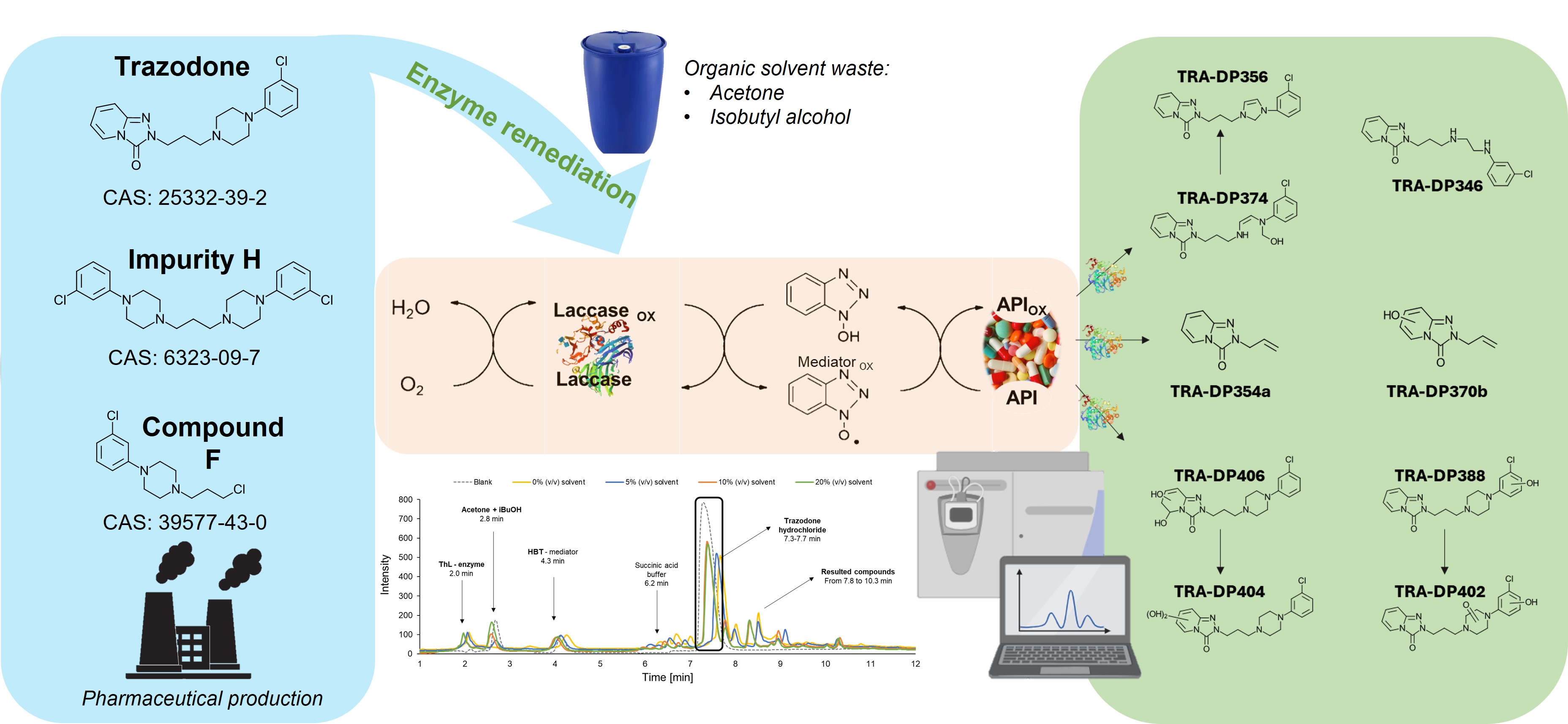
The increasing drug consumption worldwide raises environmental and health concerns, as pharmaceutical residues entering the environment pose risks to both ecosystems and human health. Enzyme remediation has been emerging in recent decades as a possible solution to eliminate recalcitrant pharmaceutical pollutants in wastewater or contaminated sites, offering a faster alternative to microbial remediation, e.g., without the need for microbial growth or adaptation. Moreover, enzymes operate under a wider range of conditions, avoiding biomass formation and disposal, and antibiotic resistance risks. In the present study, high amounts (up to 5000 mg/L) of trazodone hydrochloride and its by-products, namely Impurity H and Compound F, were enzymatically treated using a laccase-mediator system consisting of the laccase from Trametes hirsuta (ThL) and the mediator 1-hydroxybenzotriazole (HBT). Different concentrations of an organic solvent system composed of acetone and isobutyl alcohol in a 1:1 ratio and up to 20% (v/v) were employed to simulate the matrix of a real industrial production waste stream. Thereby, the enzymatic oxidation of trazodone hydrochloride and its by-products was not significantly affected by the varying solvent concentrations, resulting in maximum conversions of 62%, 73% and 62% of trazodone hydrochloride, impurity H, and impurity F, respectively. Liquid chromatography—high-resolution mass spectrometry (LC-HRMS) indicated preferential oxidation of the piperazine group in all three molecules. In vivo ecotoxicity experiments must be carried out in the future to assess the toxicological and environmental behaviour of the obtained degradation products. This work emphasised the potential role of enzymes in supporting the transition towards a more sustainable pharmaceutical industry.
- Open Access
- Article
Laccase-Mediated Transformation of Trazodone Hydrochloride and Its By-Products
- Filippo Fabbri 1, *,
- Cicely M. Warne 1,
- Palma Cassatella 1,
- David Ruso 2,
- Maria Doppler 2,
- Alessandro Pellis 1, 3,
- Georg M. Guebitz 1, 4
Author Information
Received: 06 Jun 2025 | Revised: 07 Jul 2025 | Accepted: 29 Jul 2025 | Published: 07 Aug 2025
Abstract
Graphical Abstract
Keywords
trazodone hydrochloride | enzyme remediation | pharmaceutical wastewater | laccase-mediator system
References
- 1.Graumnitz, S.; Jungmann, D. The Database ‘Pharmaceuticals in the Environment’—Update for the Period 2017–2020. 2021. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/479/publikationen/texte_163-2021_the_database_pharmaceuticals_in_the_environment.pdf (accessed on 10 July 2025).
- 2.Swan, G.; Naidoo, V.; Cuthbert, R.; et al. Removing the threat of diclofenac to critically endangered Asian vultures. PLoS Biol. 2006, 4, 395–402. https://doi.org/10.1371/journal.pbio.0040066.
- 3.European Medicine Agency. Nitrosamine Impurities Scientific Review on the Risk of Nitrosamine Impurities in Human Medicines; European Medicine Agency: Amsterdam, The Netherlands, 2018; Volume 5, pp. 1–21.
- 4.European Commission. European Union Strategic Approach to Pharmaceuticals in the Environment; European Commission: Brussels, Belgium, 2019; Volume 128, p. 13.
- 5.Wells, A.S.; Finch, G.L.; Michels, P.C.; et al. Use of enzymes in the manufacture of active pharmaceutical ingredients—A science and safety-based approach to ensure patient safety and drug quality. Org. Process Res. Dev. 2012, 16, 1986–1993. https://doi.org/10.1021/op300153b.
- 6.Bilal, M.; Iqbal, H.M.N.; Barceló, D. Perspectives on the Feasibility of Using Enzymes for Pharmaceutical Removal in Wastewater. Handb. Environ. Chem. 2021, 108, 119–143. https://doi.org/10.1007/698_2020_661.
- 7.Mir-Tutusaus, J.A.; Parladé, E; Villagrasa, M.; et al. Long-term continuous treatment of non-sterile real hospital wastewater by Trametes versicolor. J. Biol. Eng. 2019, 13, 47. https://doi.org/10.1186/s13036-019-0179-y.
- 8.Sá, H.; Michelin, M.; Tavares, T.; et al. Current Challenges for Biological Treatment of Pharmaceutical-Based Contaminants with Oxidoreductase Enzymes: Immobilization Processes, Real Aqueous Matrices and Hybrid Techniques. Biomolecules 2022, 12, 1489. https://doi.org/10.3390/biom12101489.
- 9.Somu, P.; Narayanasamy, S.; Gomez, L.A.; et al. Immobilization of enzymes for bioremediation: A future remedial and mitigating strategy. Environ. Res. 2022, 212, 113411. https://doi.org/10.1016/j.envres.2022.113411.
- 10.Slater, C.S.; Savelski, M.J. Towards a greener manufacturing environment. Innov. Pharm.Technol. 2009, 29, 78–83.
- 11.Spina, F.; Cordero, C.E.I.; Sgorbini, B.; et al. Endocrine disrupting chemicals (EDCs) in municipal wastewaters: Effective degradation and detoxification by fungal laccases. Chem. Eng. Trans. 2013, 32, 391–396. https://doi.org/10.3303/CET1332066.
- 12.Al-Sareji, O.J.; Meiczinger, M.; Salman, J.M.; et al. Ketoprofen and aspirin removal by laccase immobilized on date stones. Chemosphere 2023, 311, 137133. https://doi.org/10.1016/j.chemosphere.2022.137133.
- 13.Huber, D.; Bleymaier, K.; Pellis, A.; et al. Laccase catalyzed elimination of morphine from aqueous systems. New Biotechnol. 2018, 42, 19–25. https://doi.org/10.1016/j.nbt.2018.01.003.
- 14.Becker, D.; Della Giustina, S.V.; Rodriguez-Mozaz, S.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. https://doi.org/10.1016/j.biortech.2016.08.004.
- 15.Yang, J.; Lin, Y.; Yang, X.; et al. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazard. Mater. 2017, 322, 525–531. https://doi.org/10.1016/j.jhazmat.2016.10.019.
- 16.Alharbi, S.K.; Nghiem, L.D.; Van De Merwe, J.P.; et al. Degradation of diclofenac, trimethoprim, carbamazepine, and sulfamethoxazole by laccase from Trametes versicolor: Transformation products and toxicity of treated effluent. Biocatal. Biotransform. 2019, 37, 399–408. https://doi.org/10.1080/10242422.2019.1580268.
- 17.Zdarta, J.; Jankowska, K.; Wyszowska, M.; et al. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C 2019, 103, 109789. https://doi.org/10.1016/j.msec.2019.109789.
- 18.Ostadhadi-Dehkordi, S.; Tabatabaei-Sameni, M.; Forootanfar, H.; et al. Degradation of some benzodiazepines by a laccase-mediated system in aqueous solution. Bioresour. Technol. 2012, 125, 344–347. https://doi.org/10.1016/j.biortech.2012.09.039.
- 19.Chmelová, D.; Ondrejovič, M.; Miertuš, S. Laccases as Effective Tools in the Removal of Pharmaceutical Products from Aquatic Systems. Life 2024, 14, 230. https://doi.org/10.3390/life14020230.
- 20.Fabbri, F.; Bischof, S.; Mayr, S.; et al. The Biomodified Lignin Platform: A Review. Polymers 2023, 15, 1694. https://doi.org/10.3390/polym15071694.
- 21.Navada, K.K.; Kulal, A. Enzymatic degradation of chloramphenicol by laccase from Trametes hirsuta and comparison among mediators. Int. Biodeterior. Biodegrad. 2019, 138, 63–69. https://doi.org/10.1016/j.ibiod.2018.12.012.
- 22.Collado, N.; Rodriguez-Mozaz, S.; Gros, M.; et al. Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ. Pollut. 2014, 185, 202–212. https://doi.org/10.1016/j.envpol.2013.10.040.
- 23.Lacorte, S.; Gómez-Canela, C.; Calas-Blanchard, C. Pharmaceutical residues in senior residences wastewaters: High loads, emerging risks. Molecules 2021, 26, 5047. https://doi.org/10.3390/molecules26165047.
- 24.Giebułtowicz, J.; Nałecz-Jawecki, G. Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and in tap water in Warsaw (Poland). Ecotoxicol. Environ. Saf. 2014, 104, 103–109. https://doi.org/10.1016/j.ecoenv.2014.02.020.
- 25.Clinical.com. Trazodone Drug Usage Statistics, United States, 2013–2022. 2022. Available online: https://clincalc.com/DrugStats/Drugs/Trazodone (accessed on 1 May 2025).
- 26.IMARC. Trazodone HCl pricing Report 2024: Price Trend, Chart, Market Analysis, News, Demand, Historical and Forecast Data. 2023. Available online: https://www.imarcgroup.com/trazodone-hcl-pricing-report (accessed on 1 May 2025).
- 27.García-Zamora, J.L.; León-Aguirre, K.; Quiroz-Morales, R.; et al. Chloroperoxidase-mediated halogenation of selected pharmaceutical micropollutants. Catalysts 2018, 8, 32. https://doi.org/10.3390/catal8010032.
- 28.Henschler, D. Toxicity of Chlorinated Organic Compounds: Effects of the Introduction of Chlorine in Organic Molecules. Angew. Chem. Int. Ed. Engl. 1994, 33, 1920–1935. https://doi.org/10.1002/anie.199419201.
- 29.Iacoangeli, T.; Moro, L.M.; Torchiarolo, G.C.; et al. Continuous Process for the Preparation of Trazodone. U.S. Patent US20240139179A1, 2 May 2019.
- 30.Marchetti, M.; Iacoangeli, T.; Ciottoli, G.B.; et al. Trazodone and Trazodone Hydrochloride in Purified Form. U.S. Patent US20240139179A1, 2007.
- 31.Nyanhongo, G.S.; Gomes, J.; Gübitz, G.M.; et al. Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res. 2002, 36, 1449–1456. https://doi.org/10.1016/S0043-1354(01)00365-7.
- 32.Doppler, M.; Bueschl, C.; Ertl, F.; et al. Towards a broader view of the metabolome: Untargeted profiling of soluble and bound polyphenols in plants. Anal. Bioanal. Chem. 2022, 414, 7421–7433. https://doi.org/10.1007/s00216-022-04134-z.
- 33.Wu, M.H.; Lin, M.C.; Lee, C.C.; et al. Enhancement of laccase activity by pre-incubation with organic solvents. Sci. Rep. 2019, 9, 1–11. https://doi.org/10.1038/s41598-019-45118-x.
- 34.Dordick, J.S. Designing Enzymes for Use in Organic Solvents. Biotechnol. Prog. 1992, 8, 259–267. https://doi.org/10.1021/bp00016a001.
- 35.Mohtashami, M.; Fooladi, J.; Haddad-Mashadrizeh, A.; et al. Molecular mechanism of enzyme tolerance against organic solvents: Insights from molecular dynamics simulation. Int. J. Biol. Macromol. 2019, 122, 914–923. https://doi.org/10.1016/j.ijbiomac.2018.10.172.
- 36.Dezfouli, R.A.; Esmaeilidezfouli, E. Optimizing laccase selection for enhanced outcomes: A comprehensive review. 3 Biotech 2024, 14, 165. https://doi.org/10.1007/s13205-024-04015-5.
- 37.Abadulla, E.; Tzanov, T.; Costa, S.; et al. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 2000, 66, 3357–3362. https://doi.org/10.1128/AEM.66.8.3357-3362.2000.
- 38.Mizuno, H.; Hirai, H.; Kawai, S.; et al. Removal of estrogenic activity of iso-butylparaben and n-butylparaben by laccase in the presence of 1-hydroxybenzotriazole. Biodegradation 2009, 20, 533–539. https://doi.org/10.1007/s10532-008-9242-y.
- 39.Ashe, B.; Nguyen, L.N.; Hai, F.I.; et al. Impacts of redox-mediator type on trace organic contaminants degradation by laccase: Degradation efficiency, laccase stability and effluent toxicity. Int. Biodeterior. Biodegrad. 2016, 113, 169–176. https://doi.org/10.1016/j.ibiod.2016.04.027.
- 40.Rebrikov, D.N.; Stepanova, E.V.; Koroleva, O.V.; et al. Laccase of the lignolytic fungus Trametes hirsuta: Purification and characterization of the enzyme, and cloning and primary structure of the gene. Appl. Biochem. Microbiol. 2006, 42, 564–572. https://doi.org/10.1134/S0003683806060068.
- 41.Hegde, R.N.; Shetti, N.P.; Nandibewoor, S.T. Electro-oxidation and determination of trazodone at multi-walled carbon nanotube-modified glassy carbon electrode. Talanta 2009, 79, 361–368. https://doi.org/10.1016/j.talanta.2009.03.064.
- 42.Fabbrini, M.; Galli, C.; Gentili, P. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Catal. B Enzym. 2002, 16, 231–240. https://doi.org/10.1016/S1381-1177(01)00067-4.
- 43.Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; et al. Photocatalytic degradation of amitriptyline, trazodone and venlafaxine using modified cobalt-titanate nanowires under UV–Vis radiation: Transformation products and in silico toxicity. Chem. Eng. J. 2019, 373, 1338–1347. https://doi.org/10.1016/j.cej.2019.05.137.
- 44.Osawa, R.A.; Monteiro, O.C.; Oliveira, M.C.; et al. Comparative study on photocatalytic degradation of the antidepressant trazodone using (Co, Fe and Ru) doped titanate nanowires: Kinetics, transformation products and in silico toxicity assessment. Chemosphere 2020, 259, 127486. https://doi.org/10.1016/j.chemosphere.2020.127486.
- 45.Thummar, M.; Patel, P.N.; Kushwah, B.S.; et al. Application of the UHPLC method for separation and characterization of major photolytic degradation products of trazodone by LC-MS and NMR. New J. Chem. 2018, 42, 16972–16984. https://doi.org/10.1039/c8nj03545h.
- 46.Mathur, P.; Kochar, M.; Conlan, X.A.; et al. Laccase mediated transformation of fluoroquinolone antibiotics: Analyzing degradation pathways and assessing algal toxicity. Environ. Pollut. 2024, 360, 124700. https://doi.org/10.1016/j.envpol.2024.124700.
How to Cite
Fabbri, F.; Warne, C. M.; Cassatella, P.; Ruso, D.; Doppler, M.; Pellis, A.; Guebitz, G. M. Laccase-Mediated Transformation of Trazodone Hydrochloride and Its By-Products. Renewable Chemistry 2025, 1 (1), 4. https://doi.org/10.53941/rc.2025.100004.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


