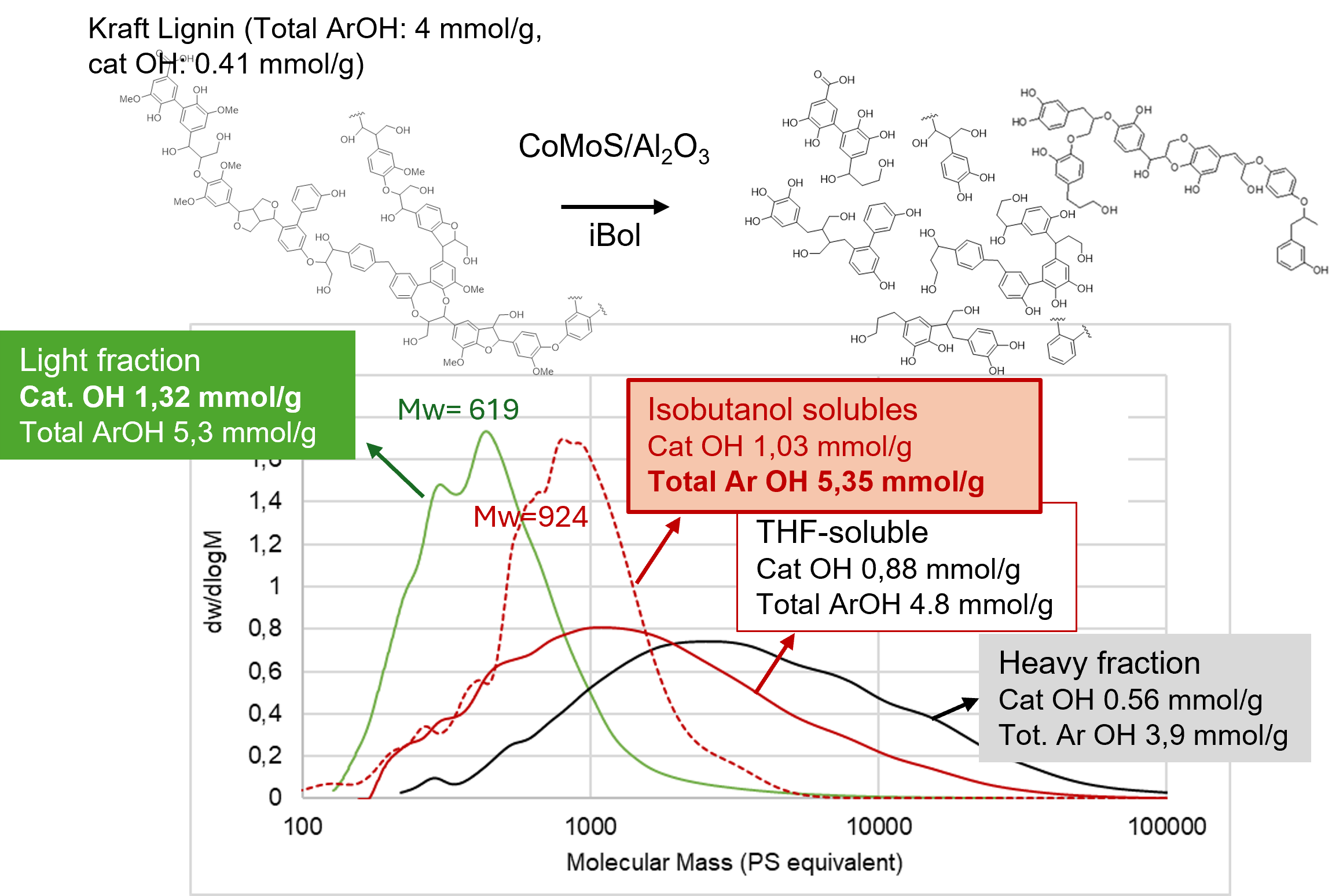
The catalytic upgrading of technical lignin into valuable functional materials represents a key challenge for sustainable chemistry. This work presents a one-pot hydrogenolysis and demethylation process to transform Kraft lignin into hydroxyl (OH)-enriched oligomers suitable for bio-based polymer applications. Hydrogenolysis reactions were conducted using isobutanol as solvent and heterogeneous catalysts: CoMo(Ox/S)/Al2O3, CoMo(Ox/S)/ZrO2, Mo2C/C, and Pd/C. Products mixtures were separated into isobutanol soluble/insoluble and light/heavy fractions, and characterized by 31P NMR, 13C NMR, HSQC, and SEC. Among the selected candidates, alumina-supported CoMoS and CoMoOx were the most efficient. CoMoS/Al2O3 enhanced demethylation, producing catechol-type OH groups while CoMoOx/Al2O3 favored depolymerization, generating more soluble oligomers but with lower demethylation. Light fractions were especially enriched in guaiacyl and catechol groups, while heavier ones retained more condensed C5 structures. These results confirm that demethylation and limited depolymerization (IUB ether bond cleavage) can be achieved, using a typical CoMoS/Al2O3 catalyst under hydrogenolysis conditions, primarily affecting smaller, soluble lignin-derivatives fragments. These fractions are promising intermediates for bio-based polyol for polymer synthesis, providing a sustainable route to valorize technical lignins using conventional hydrotreating catalysts.
- Open Access
- Article
One-Pot Technical Lignin Catalytic Depolymerization and Demethylation towards Valuable OH-Enriched Oligomers
Author Information
Received: 05 Aug 2025 | Revised: 16 Sep 2025 | Accepted: 07 Nov 2025 | Published: 14 Nov 2025
Abstract
Graphical Abstract
Keywords
lignin | hydrogenolysis | catechol | catalysis | oligomers
References
- 1.Sethupathy, S.; Morales, G.M.; Gao, L.; et al. Lignin valorization: Status, challenges and opportunities. Biores. Tech. 2022, 347, 126696. https://doi.org/10.1016/j.biortech.2022.126696.
- 2.Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; et al. A concise review of current lignin production, applications, products and their environmental impact. J. Ind. Crops 2019, 139, 111526. https://doi.org/10.016/j.indcrop.2019.111526.
- 3.Zijlstra, D.S.; Analbers, C.A.; de Korte, J.; et al. Efficient Mild Organosolv Lignin Extraction in a Flow-Through Setup Yielding Lignin with High β-O-4 Content. Polymers 2019, 11, 1913. https://doi.org/10.3390/polym11121913.
- 4.Renders, T.; Van den Bossche, G.; Vangeel, T.; et al. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Op. Biotech. 2019, 56, 193–201. https://doi.org/10.1016/j.copbio.2018.12.005.
- 5.Khorshidi, F.H.; Najafi, S.K.; Najafi, F.; et al. The extraction of polyol for the synthesis of lignin-based polyurethane coatings—A review. Wood Mater. Sci. Eng. 2024, 19, 794–802. https://doi.org/10.1080/17480272.2024.2335502.
- 6.Luo, Z.; Liu, C.; Radu, A.; et al. Carbon–carbon bond cleavage for a lignin refinery. Nat. Chem. Eng. 2024, 1, 61–72. https://doi.org/10.1038/s44286-023-00006-0.
- 7.Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Progr. Polym. Sc. 2014, 39, 1266–1290. https://doi.org/10.1016/j.progpolymsci.2013.11.004.
- 8.Wybo, N.; Duval, A.; Avérous, L. Benign and selective amination of lignins towards aromatic biobased building blocks with primary amines. Angew. Chem. Int. Ed. 2024, 63, e202403806. https://doi.org/10.1002/anie.202403806.
- 9.Wybo, N.; Cherasse, E.; Duval, A.; et al. Unlocking sustainable, aromatic, and versatile materials through transurethanization: Development of non-isocyanate polyurethanes from lignins. Mater. Chem. A 2025, 13, 11557–11572. https://doi.org/10.1039/d4ta08582e.
- 10.Pu, J.; Nguyen, T.-S.; Leclerc, E.; et al. Lignin catalytic hydroconversion in a semi-continuous reactor: An experimental study. Appl. Catal. B Env. 2019, 256, 117769. https://doi.org/10.1016/j.apcatb.2019.117769.
- 11.Kosyakov, D.S.; Pikovskoi, I.I.; Ul’yanovskii, N.V. Dopant-assisted atmospheric pressure photoionization Orbitrap mass spectrometry–An approach to molecular characterization of lignin oligomers. Anal. Chim. Acta 2021, 1179, 338836. https://doi.org/10.1016/j.aca.2021.338836.
- 12.Letourneau, D.R.; Volmer, D.A. Mass spectrometry‐based methods for the advanced characterization and structural analysis of lignin: A review. Mass Spec. Rev. 2023, 42, 144–188. https://doi.org/10.1002/mas.21716.
- 13.Sander, K.; Dütsch, L.; Bremer, M.; et al. Characterization of soluble and insoluble lignin oligomers by means of ultrahigh resolving mass spectrometry. Energy Fuels 2023, 37, 439−449. https://doi.org/10.1021/acs.energyfuels.2c03538.
- 14.Bartolomei, E.; Le Brech, Y.; Dufour, A.; et al. Lignin depolymerization: A comparison of methods to analyze monomers and oligomers. ChemSusChem 2020, 13, 4633. https://doi.org/10.1002/cssc.202001126.
- 15.Alherech, M.; Omolabake, S.; Holland, C.M.; et al. From lignin to valuable aromatic chemicals: Lignin depolymerization and monomer separation via centrifugal partition chromatography. ACS Cent. Sci. 2021, 7, 1831–1837. https://doi.org/10.1021/acscentsci.1c00729.
- 16.Tammekivi, E.; Batteau, M.; Laurenti, D.; et al. A powerful two-dimensional chromatography method for the non-target analysis of depolymerised lignin. Anal. Chim. Acta 2024, 1288, 342157. https://doi.org/10.1016/j.aca.2023.342157.
- 17.Tammekivi, E.; Lilti, H.; Batteau, M.; et al. Complementarity of two-dimensional gas chromatography and two-dimensional liquid chromatography for the analysis of depolymerised lignin. J. Chrom. A 2024, 1736, 465401. https://doi.org/10.1016/j.chroma.2024.465401.
- 18.Ferhan, M.; Yan, N.; Sain, M. A new method for demethylation of lignin from woody biomass using biophysical methods. J. Chem. Eng. Process. Techn. 2013, 4, 160. https://doi.org/10.4172/2157-7048.1000160.
- 19.Kozmelj, T.R.; Bartolomei, E.; Dufour, A.; et al. Oligomeric fragments distribution, structure and functionalities upon ruthenium-catalyzed technical lignin depolymerization. Biomass Bioenergy 2024, 181, 107056. https://doi.org/10.1016/j.biombioe.2024.107056.
- 20.Liu, X.; Jiang, Z.; Feng, S.; et al. Catalytic depolymerization of organosolv lignin to phenolic monomers and low molecular weight oligomers. Fuel 2019, 244, 247–257. https://doi.org/10.1016/j.fuel.2019.01.117.
- 21.Karnitski, A.; Choi, J.-W.; Suh, D.J.; et al. Roles of metal and acid sites in the reductive depolymerization of concentrated lignin over supported Pd catalysts. Catal. Today 2023, 113844, 411–412. https://doi.org/10.1016/j.cattod.2022.07.012.
- 22.Wu, X.; Liao, Y.; Bomon, J.; et al. Lignin‐first monomers to catechol: Rational cleavage of C−O and C−C bonds over zeolites. ChemSusChem 2022, 15, e202102248. https://doi.org/10.1002/cssc.202102248.
- 23.Ji, N.; Wang, Z.; Diao, X.; et al. Highly selective demethylation of anisole to phenol over H 4 Nb 2 O 7 modified MoS 2 catalyst. Catal. Sci. Technol. 2021, 11, 800–809. https://doi.org/10.1039/D0CY01972K.
- 24.Jiang, L.; Guo, H.; Li, C.; et al. Selective cleavage of lignin and lignin model compounds without external hydrogen, catalyzed by heterogeneous nickel catalysts. Chem. Sci. 2019, 10, 4458–4468. https://doi.org/10.1039/C9SC00691E.
- 25.Podschun, J.; Saake, B.; Lehnen, R. Catalytic demethylation of organosolv lignin in aqueous medium using indium triflate under microwave irradiation. React. Funct. Polym. 2017, 119, 82–86. https://doi.org/10.1016/j.reactfunctpolym.2017.08.007.
- 26.Bui, V.N.; Laurenti, D.; Delichère, P.; et al. Hydrodeoxygenation of guaiacol. Part II: Support effect for CoMoS catalysts on HDO activity and selectivity. Appl. Cat. B Env. 2011, 101, 246–255. https://doi.org/10.1016/j.apcatb.2010.10.031.
- 27.Meng, S.; Xue, X.; Wenig, Y.; et al. Synthesis and characterization of molybdenum carbide catalysts on different carbon supports. Cata. Today 2022, 402, 266–275. https://doi.org/10..1016/j.cattod.2022.04.020.
- 28.Meng, X.; Crestini, C.; Ben, H.; et al. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. https://doi.org/10.1038/s41596-019-0191-1.
- 29.Smit, A.T.; Dezaire, T.; Riddell, L.A.; et al. Reductive partial depolymerization of acetone organosolv lignin to tailor lignin molar mass, dispersity, and reactivity for polymer applications. ACS Sustain. Chem. Eng. 2023, 11, 6070–6080. https://doi.org/10.1021/acssuschemeng.3c00617.
- 30.Van Aelst, K.; Van Sinay, E.; Vangeel, T.; et al. Reductive catalytic fractionation of pine wood: Elucidating and quantifying the molecular structures in the lignin oil. Chem. Sci. 2020, 11, 11498–11508. https://doi.org/10.1039/D0SC04182C.
- 31.Joffres, B.; Lorentz, C.; Vidalie, M.; et al. Catalytic hydroconversion of a wheat straw soda lignin: Characterization of the products and the lignin residue. Appl. Cat. B Env. 2014, 145, 167–176. https://doi.org/10.1016/j.apcatb.2013.01.039.
- 32.Oregui-Bengoechea, M.; Gandarias, I.; Arias, P.L.; et al. Unraveling the role of formic acid and the type of solvent in the catalytic conversion of lignin: A holistic approach. ChemSusChem 2017, 10, 754–766. https://doi.org/10.1002/cssc.201601410.
- 33.Wu, X.; Smet, E.; Brandi, F.; et al. Advancements and perspectives toward lignin valorization via O‐demethylation. Angew. Chem. Int. Ed. 2023, 136, e202317257. https://doi.org/10.1002/anie.202317257.
- 34.Chen, P.; Zhang, Q.; Shu, R.; et al. Catalytic depolymerization of the hydrolyzed lignin over mesoporous catalysts. Bior. Tech. 2017, 226, 125–131. https://doi.org/10.1016/j.biortech.2016.12.030.
- 35.Kloekhorst, A.; Heeres, H.J. Catalytic hydrotreatment of alcell lignin using supported Ru, Pd, and Cu catalysts. ACS Sust. Chem. Eng. 2015, 3, 1905–1914. https://doi.org/10.1021/acssuschemeng.5b00041.
- 36.Yang, X.; Feng, M.; Choi, J.-S.; et al. Depolymerization of corn stover lignin with bulk molybdenum carbide catalysts. Fuel 2019, 244, 528–535. https://doi.org/10.1016/j.fuel.2019.02.023.
- 37.Bui, V.N.; Laurenti, D.; Afanasiev, P.; et al. Hydrodeoxygenation of guaiacol with CoMo catalysts. Part I: Promoting effect of cobalt on HDO selectivity and activity. Appl. Cat. B Env. 2011, 101, 239–245. https://doi.org/10.1016/j.apcatb.2010.10.025.
- 38.Mañas, A.H.; Vilcocq, L.; Fongarland, P.; et al. Lignin catalytic oxidation by CuO/TiO2: Role of catalyst in phenolics formation. Waste Biom. Valor. 2023, 14, 3789–3809. https://doi.org/10.1007/s12649-023-02082-y.
- 39.Huda, M.M.; Rai, N. Effect of solvent on the interaction of lignin with a zeolite nanosheet in the condensed phase. J. Phys. Chem. B 2023, 127, 6767–6777. https://doi.org/10.1021/acs.jpcb.3c02085.
How to Cite
Lilti, H.; Olivier, L.; Lorentz, C.; Geantet, C.; Laurenti, D. One-Pot Technical Lignin Catalytic Depolymerization and Demethylation towards Valuable OH-Enriched Oligomers. Renewable Chemistry 2025, 1 (1), 5. https://doi.org/10.53941/rc.2025.100005.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


