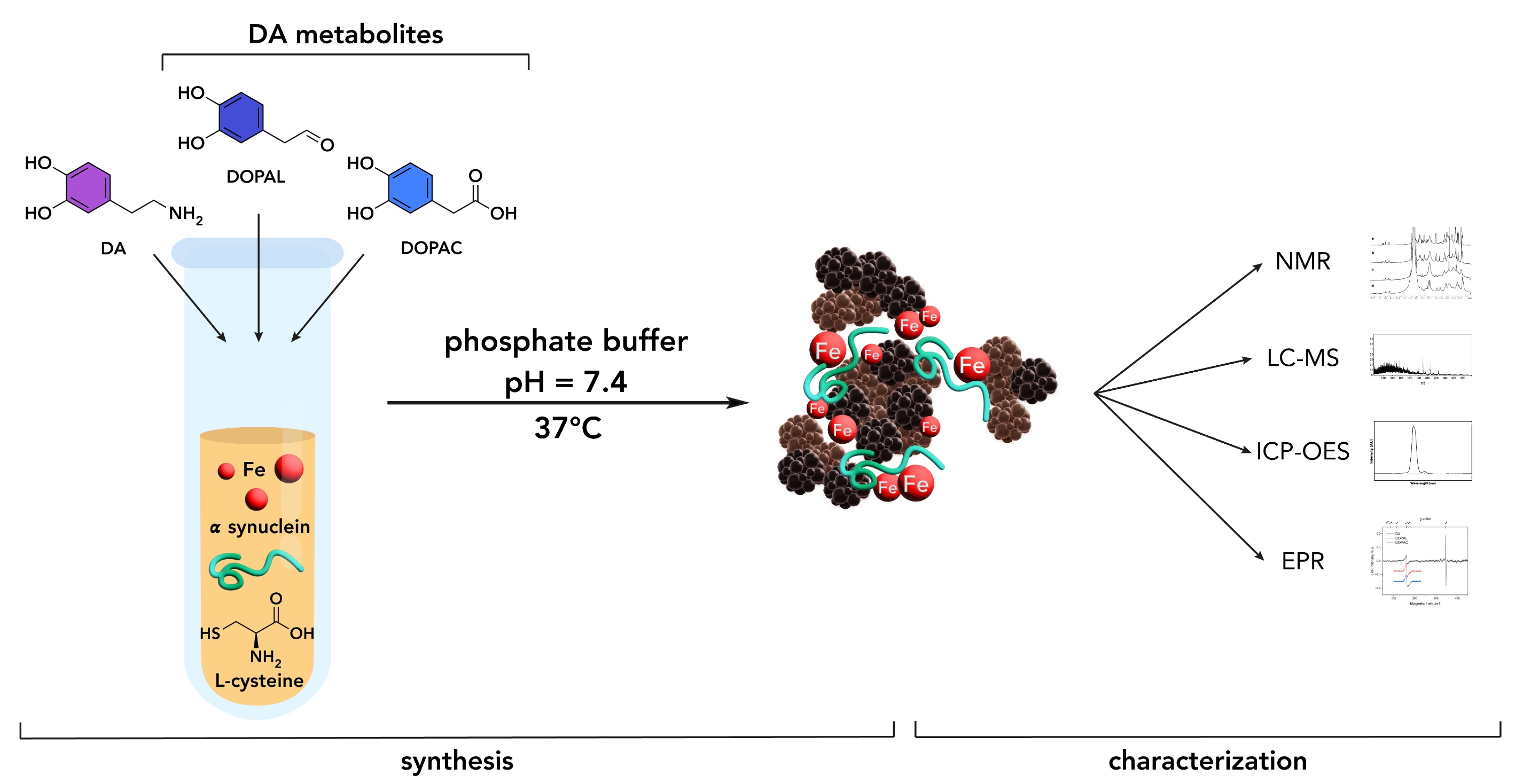
Melanin-protein-Fe conjugates serving as new synthetic analogues of brain neuromelanin were prepared by oxidative oligomerization of dopamine (DA) and its metabolites, 3,4-dihydroxyphenyl acetaldehyde (DOPAL) and 3,4-dihydroxyphenylacetic acid (DOPAC) in the presence of α-synuclein (αSyn), L-cysteine, and iron salts. The initial reaction of cysteine with the quinone derivatives of the catechols yields the corresponding cysteinyl-catechols which under iron promoted oxidative conditions convert to cysteinyl-quinones that undergo nucleophilic addition by the side chain of the protein lysines and tyrosines. In the case of dopamine, this reaction is in competition with internal cyclization by the amino group to generate melanochrome so that α-synuclein was found partially unmodified and non-covalently bound to the melanin moiety. For DOPAL and DOPAC the functional groups on the catechol side chains can further stabilize the melanin-protein conjugates through condensation or charge interaction with the several lysines present in αSyn. The presence of cysteine in the melanin component allows to classify the resulting conjugates as pheomelanin-αSyn-Fe derivatives of DA, DOPAL and DOPAC. The iron ions acting as catalysts are initially bound to the catechol groups and remain entrapped into the melanin moieties mostly associated in clusters, with less than 10% present as mononuclear centers. The pheomelanin-αSyn-Fe conjugates are soluble and were characterized by NMR, LC-MS, EPR and ICP-OES. The present results show the possible mechanism of incorporation of αSyn into brain neuromelanin.
- Open Access
- Article
α-Synuclein and Dopamine Metabolites DOPAL and DOPAC: A Pathway to New Synthetic Neuromelanin Models
- Andrea Capucciati 1, 2,
- Michela Sturini 1,
- Stefania Nicolis 1,
- Fabio A. Zucca 2,
- Marco Bisaglia 3,
- Giulia Favetta 3,
- Marco Bortolus 4,
- Luigi Zecca 2, 5,
- Gianni Pezzoli 5,
- Luigi Casella 1,
- Enrico Monzani 1, *
Author Information
Received: 28 Mar 2025 | Revised: 30 Apr 2025 | Accepted: 06 May 2025 | Published: 09 May 2025
Abstract
Graphical Abstract
Keywords
neuromelanin | alpha-synuclein | DOPAL | DOPAC | melanin-protein conjugates | iron complexes
References
- 1.Xu,; Nussinov, R.; Ma, B. Coupling of the non-amyloid-component (NAC) domain and the KTK(E/Q)GV repeats stabilize the α-synuclein fibrils. Eur. J. Med. Chem. 2016, 121, 841–850.
- 2.Morris,R.; Spillantini, M.G.; Sue, C.M.; et al. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304.
- 3.Bartels, A traffic jam leads to Lewy bodies. Nat. Neurosci. 2019, 22, 1039–1045.
- 4.Calabresi,; Di Lazzaro, G.; Marino, G.; et al. Advances in understanding the function of alpha-synuclein: Implications for Parkinson’s disease. Brain 2023, 146, 3587–3597.
- 5.Lin,J.; Chen, S.D.; Lin, K.L.; et al. Iron brain menace: The involvement of ferroptosis in Parkinson disease. Cells 2022, 11, 3829.
- 6.Gaeta,; Hider, R.C. The crucial role of metal ions in neurodegeneration: The basis for a promising therapeutic strategy. Br. J. Pharmacol. 2005, 146, 1041–1059.
- 7.Brooks,; Everett, J.; Lermyte, F.; et al. Analysis of neuronal iron deposits in Parkinson’s disease brain tissue by synchrotron x-ray spectromicroscopy. J. Trace Elem. Med. Biol. 2020, 62, 126555.
- 8.Goedert, Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001, 2, 492–501.
- 9.Yavich,; Tanila, H.; Vepsäläinen, S.; et al. Role of alpha-synuclein in presynaptic dopamine recruitment. J. Neurosci. 2004, 24, 11165–11170.
- 10.Plotegher,; Greggio, E.; Bisaglia, M.; et al. Biophysical ground work as a hinge to unravel the biology of α-synuclein aggregation and toxicity. Q. Rev. Biophys. 2014, 47, 1–48.
- 11.Monzani,; Nicolis, S.; Dell’Acqua, S.; et al. Dopamine, oxidative stress and protein-quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed., 2019, 58, 6512–6527.
- 12.Lehrer,; Rheinstein, P.H. α Synuclein enfolds tyrosine hydroxylase and dopamine ß hydroxylase, potentially reducing dopamine and norepinephrine synthesis. J. Proteins Proteom. 2022, 13, 109–115.
- 13.Nagatsu,; Levitt, M.; Udenfriend, S.; Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J. Biol. Che. 1964, 239, 2910–2917.
- 14.Nagatsu,; Nakashima, A.; Watanabe, H.; et al. The role of tyrosine hydroxylase as a key player in neuromelanin synthesis and the association of neuromelanin with Parkinson’s disease. J. Neural Transm. 2023, 130, 611–625.
- 15.Beard,; Erikson, K.M.; Jones, B.C. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J. Nutr. 2003, 133, 1174–1179.
- 16.Flydal,I.; Martinez, A. Phenylalanine hydroxylase: Function, structure, and regulation. IUBMB Life 2013, 65, 341–349.
- 17.Wu,P.; Kim, S.; Fela, D.A.; et al. Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: Implication for aggregation. J. Mol. Biol. 2008, 378, 1104–1115.
- 18.Binolfi,; Rasia, R.M.; Bertoncini, C.W.; et al. Interaction of alpha-synuclein with divalent metal ions reveals key differences: A link between structure, binding specificity and fibrillation enhancement. J. Am. Chem. Soc. 2006, 128, 9893–9901.
- 19.Lorentzon,; Kumar, R.; Horvath, I.; et al. Differential effects of Cu2+ and Fe3+ ions on in vitro amyloid formation of biologically-relevant a-synuclein variants. Biometals 2020, 33, 97–106.
- 20.McDowall,S.; Brown, D.R. Alpha-synuclein: Relating metals to structure, function and inhibition. Metallomics 2016, 8, 385–397.
- 21.Sian-Hulsmann,; Riederer, P. The role of alpha-synuclein as ferrireductase in neurodegeneration associated with Parkinson’s disease. J. Neural Transm. 2020, 127, 749–754.
- 22.Zucca,A.; Vanna, R.; Cupaioli, F.A.; et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson’s disease. NPJ Park. Dis. 2018, 4, 17.
- 23.Zecca,; Bellei, C.; Costi, P.; et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc. Natl. Acad. Sci. USA 2008, 105, 17567–17572.
- 24.Zucca,A.; Segura-Aguilar, J.; Ferrari, E.; et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119.
- 25.Xu,; Chan, P. Interaction between neuromelanin and alpha-synuclein in Parkinson’s disease. Biomolecules 2015, 5, 1122–1142.
- 26.Xuan,; Xu, S.L.; Lu, D.H.; et al. Increased expression of α-synuclein in aged human brain associated with neuromelanin accumulation. J. Neural Transm. 2011, 118, 1575–1583.
- 27.Nagatsu,; Nakashima, A.; Wakamatsu, K. Neuromelanin in Parkinson’s disease: Tyrosine hydroxylase and tyrosinase. Int. J. Mol. Sci. 2022, 23, 4176.
- 28.Wakamatsu,; Tanaka, H.; Tabuchi, K.; et al. Reduction of the nitro group to amine by hydroiodic acid to synthesize o-aminophenol derivatives as putative degradative markers of neuromelanin. Molecules 2014, 19, 8039–8050.
- 29.Jinsmaa,; Cooney, A.; Sullivan, P.; et al. The serotonin aldehyde, 5-HIAL, oligomerizes alpha-synuclein. Neurosci. Lett. 2015, 590, 134–137.
- 30.Plotegher,; Berti, G.; Ferrari, E.; et al. DOPAL derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci. Rep. 2017, 7, 40699.
- 31.Carmo-Gonçalves,; do Nascimento, L.A.; Cortines, J.R.; et al. Exploring the role of methionine residues on the oligomerization and neurotoxic properties of DOPAL-modified α-synuclein. Biochem. Biophys. Res. Commun. 2018, 505, 295–301.
- 32.He,; Wang, F.; Yung, K.K.L.; et al. Effects of α-synuclein-associated post-translational modifications in Parkinson’s disease. ACS Chem. Neurosci. 2021, 12, 1061–1071.
- 33.Plotegher,; Bubacco, L. Lysines, Achilles’ heel in alpha-synuclein conversion to a deadly neuronal endotoxin. Aging Res. Rev. 2016, 26, 62–71.
- 34.Zhou,; Gallagher, A.; Hong, D.-P.; et al. At low concentrations, 3,4-dihydroxyphenylacetic acid (DOPAC) binds non-covalently to alpha-synuclein and prevents its fibrillation. J. Mol. Biol. 2009, 388, 597–610.
- 35.Ferrari,; Engelen, M.; Monzani, E.; et al. Synthesis and structural characterization of soluble neuromelanin analogs provides important clues to its biosynthesis. J. Biol. Inorg. Chem. 2013, 18, 81–93.
- 36.Ferrari,; Capucciati, A.; Prada, I.; et al. Synthesis, structure characterization, and evaluation in microglia cultures of neuromelanin analogues suitable for modeling Parkinson’s disease. ACS Chem. Neurosci. 2017, 8, 501–512.
- 37.Capucciati,; Monzani, E.; Sturini, M.; et al. Water-soluble melanin–protein–Fe/Cu conjugates derived from norepinephrine as reliable models for neuromelanin of human brain locus coeruleus. Angew. Chem. Int. Ed. 2022, 61, e202204787.
- 38.Ito, Encapsulation of a reactive core in neuromelanin. Proc. Natl. Acad. Sci. USA 2006, 103, 14647–14648.
- 39.Bush,D.; Garguilo, J.; Zucca, F.A.; et al. The surface oxidation potential of human neuromelanin reveals a spherical architecture with a pheomelanin core and a eumelanin surface. Proc. Natl. Acad. Sci. USA 2006, 103, 14785–14789.
- 40.Wise,M.; Wagener, A.; Fietzek, U.M.; et al. Interactions of dopamine, iron, and alpha-synuclein linked to dopaminergic neuron vulnerability in Parkinson’s disease and neurodegeneration with brain iron accumulation disorders. Neurobiol. Dis. 2022, 175, 105920.
- 41.Robbins,H. Preparation and properties of p-hydroxyphenylacetaldehyde and 3-methoxy-4-hydroxyphenylacetaldehyde. Arch. Biochem. Biophys. 1966, 114, 576–584.
- 42.Bou-Abdallah,; Chasteen, N.D. Spin concentration measurements of high-spin (g’ = 4.3) rhombic iron(III) ions in biological samples: Theory and application. J. Biol. Inorg. Chem. 2008, 13, 15–24.
- 43.Ito,; Sugumaran, M.; Wakamatsu, K. Chemical reactivities of ortho-quinones produced in living organisms: Fate of quinonoids produced by tyrosinase and phenoloxidase action on phenols and catechols. Int. J. Mol. Sci. 2020, 21, 6080.
- 44.Wakamatsu,; Nakao, K.; Tanaka, H.; et al. The oxidative pathway to dopamine–protein conjugates and their pro-oxidant activities: Implications for the neurodegeneration of Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 2575.
- 45.Zucca,A.; Capucciati, A.; Bellei, C.; et al. Neuromelanins in brain aging and Parkinson’s disease: Synthesis, structure, neuroinflammatory, and neurodegenerative role. IUBMB Life 2023, 75, 55–65. https://doi.org/10.1002/iub.2654.
- 46.Arosio,; Elia, L.; Poli, M. Ferritin, cellular iron storage and regulation. IUBMB Life 2017, 69, 414–422.
- 47.Zecca,; Shima, T.; Stroppolo, A.; et al. Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience 1996, 73, 407–415.
- 48.Zecca,; Stroppolo, A.; Gatti, A.; et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. USA 2004, 101, 9843–9848.
- 49.Capucciati,; Zucca, F.A.; Monzani, E.; et al. Interaction of neuromelanin with xenobiotics and consequences for neurodegeneration; promising experimental models. Antioxidants 2021, 10, 824.
Issue
Volume 1, Issue 1How to Cite
Capucciati, A.; Sturini, M.; Nicolis, S.; Zucca, F. A.; Bisaglia, M.; Favetta, G.; Bortolus, M.; Zecca, L.; Pezzoli, G.; Casella, L.; Monzani, E. α-Synuclein and Dopamine Metabolites DOPAL and DOPAC: A Pathway to New Synthetic Neuromelanin Models. Bioinorganics and Biocatalysis 2026, 1 (1), 2.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


