Thrombin, a serine protease, is a critical biomarker for blood-related diseases, with its serum levels playing a key role in clinical diagnosis. In this study, we developed a dual-aptamer-based, dual-mode Förster resonance energy transfer (FRET) lateral flow strip for the rapid and sensitive detection of thrombin. Quantum dots (QDs) were employed as the energy donor, while gold nanoparticles (AuNPs) served as the acceptor. Upon thrombin binding, the colorimetric signal from AuNPs and the fluorescent signal from QDs exhibited distinct variations, each acting as an independent indicator for precise quantification. Using this dual-mode FRET lateral flow strip, thrombin was successfully detected in serum samples, achieving a fluorescence-based detection limit of 0.135 nM-twice as sensitive as the colorimetric method. The integration of FRET and lateral flow strip technologies provides a robust platform for precise biomarker detection and significantly enhances the performance of colorimetric assays.
- Open Access
- Article
Dual-Mode Lateral Flow Strip with Förster Resonance Energy Transfer for Rapid and Accurate Thrombin Detection
- Qi Chen 1,
- Li Yao 1, 2,
- Wei Qu 1,
- Haoyang Xu 1,
- Chao Yan 1,
- Jianguo Xu 1, 3,
- Wei Chen 1, 4, *
Author Information
Received: 09 Mar 2025 | Revised: 27 Jun 2025 | Accepted: 01 Jul 2025 | Published: 15 Jul 2025
Abstract
Graphical Abstract
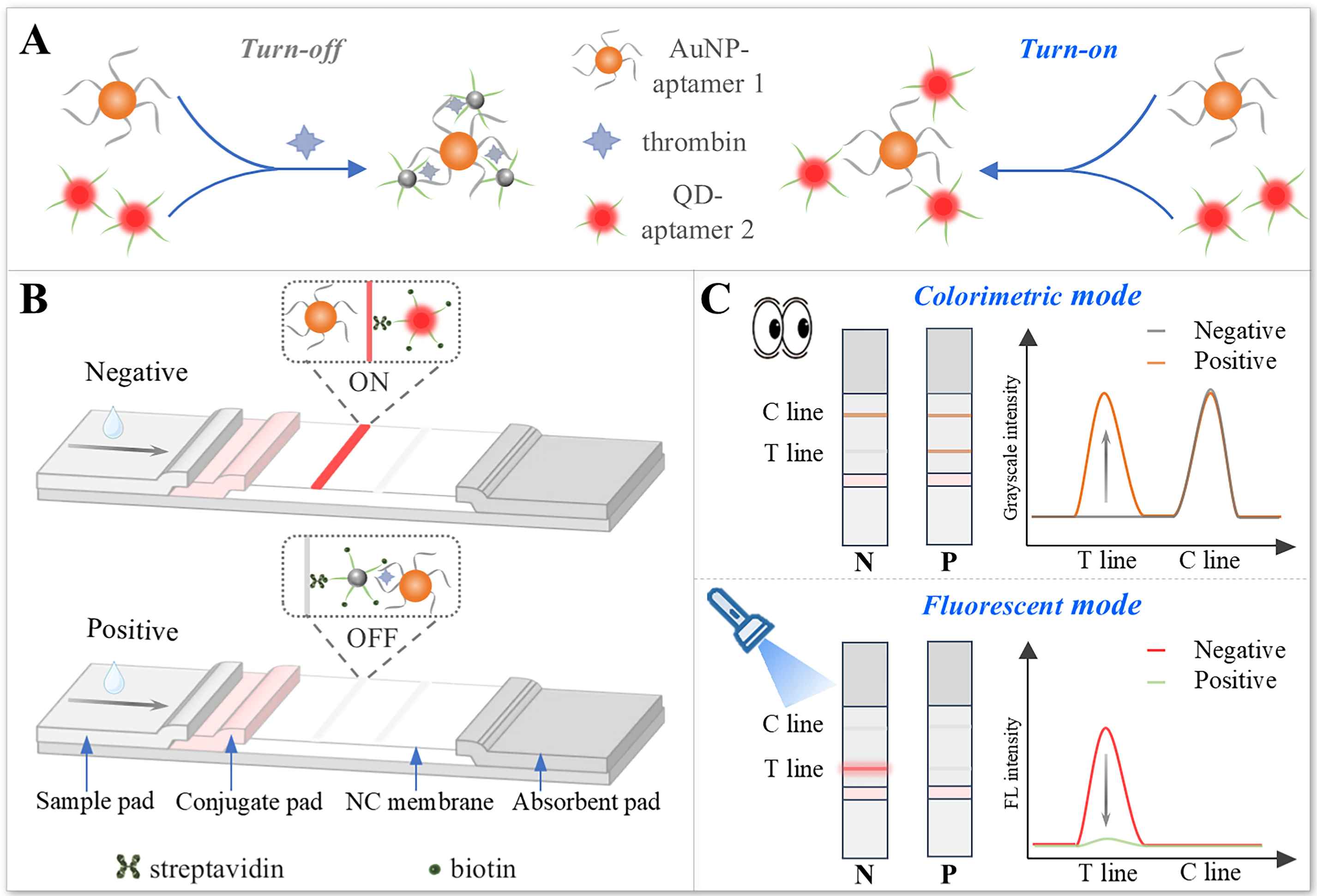
Keywords
dual-aptamer | Förster resonance energy transfer (FRET) | lateral flow strip | thrombin | dual-mode
References
- 1.Hattori, M.; Sugiura, N.; Wazawa, T.; et al. Ratiometric Bioluminescent Indicator for a Simple and Rapid Measurement of Thrombin Activity Using a Smartphone. Anal. Chem. 2021, 93, 13520–13526.
- 2.Popović, M.; Smiljanić, K.; Dobutović, B.; et al. Thrombin and vascular inflammation. Mol. Cell. Biochem. 2012, 359, 301–313.
- 3.Han, C.; Yuan, X.; Shen, Z.; et al. A paper-based lateral flow sensor for the detection of thrombin and its inhibitors. Anal. Chim. Acta 2022, 1205, 339756.
- 4.Liu, X.H.; Ba, R.Y.; Wang, W.H.; et al. Roles of nanomaterials in thrombin detection. TrAC-Trends Anal. Chem. 2024, 175, 117734.
- 5.Ye, F.; Garton, H.J.L.; Hua, Y.; et al. The Role of Thrombin in Brain Injury After Hemorrhagic and Ischemic Stroke. Transl. Stroke Res. 2021, 12, 496–511.
- 6.Liu, Z.W.; Ma, N.; Yu, S.B.; et al. Hemin-catalyzed SI-RAFT polymerization for thrombin detection. Microchem. J. 2023, 189, 108521.
- 7.Troisi, R.; Balasco, N.; Autiero, I.; et al. Exosite Binding in Thrombin: A Global Structural/Dynamic Overview of Complexes with Aptamers and Other Ligands. Int. J. Mol. Sci. 2021, 22, 10803.
- 8.Ning, J.; Bao, X.; Chen, H.; et al. A highly sensitive and specific fluorescent probe for thrombin detection and high-throughput screening of thrombin inhibitors in complex matrices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 325, 125136.
- 9.Samani, S.S.; Sameiyan, E.; Yazdi, F.T.; et al. Sandwich-type aptamer-based biosensors for thrombin detection. Anal. Methods-UK 2024, 16, 1985–2001.
- 10.Soni, G.K.; Wangoo, N.; Sharma, R.K. A smartphone-based ultrasensitive colorimetric aptasensing platform for serine protease thrombin detection. Microchem. J. 2024, 199, 109906.
- 11.Zhang, J.; Xiang, J.; Liao, L.; et al. Proximity binding-initiated DNA walker and CRISPR/Cas12a reaction for dual signal amplification detection of thrombin. Talanta 2023, 256, 124286.
- 12.Huang, Y.; Li, S.; Liu, C.; et al. One-step competitive assay for detection of thrombin via disassembly of diblock oligonucleotide functionalised nanogold aggregates. Sensor Actuat. B-Chem. 2023, 376, 133032.
- 13.Sharma, R.; Waller, A.P.; Agrawal, S.; et al. Thrombin-Induced Podocyte Injury Is Protease-Activated Receptor Dependent. J. Am. Soc. Nephrol. 2017, 28, 2619–2631.
- 14.Raucci, A.; Sorrentino, G.; Singh, S.; et al. Cost-effective, user-friendly detection and preconcentration of thrombin on a sustainable paper-based electrochemical platform. Anal. Bioanal. Chem. 2025, 417, 1863–1872.
- 15.Wang, Y.; Liu, C.; Zhao, W.; et al. Biosensors and Biomarkers for the Detection of Motion Sickness. Adv. Healthc. Mater. 2025, 14, e2403606.
- 16.Vairaperumal, T.; Huang, C.C.; Liu, P.Y. Optical Nanobiosensor-Based Point-of-Care Testing for Cardiovascular Disease Biomarkers. ACS Appl. Bio Mater. 2023, 6, 2591–2613.
- 17.Dong, T.; Yu, C.; Mao, Q.; et al. Advances in biosensors for major depressive disorder diagnostic biomarkers. Biosens. Bioelectron. 2024, 258, 116291.
- 18.Klebes, A.; Ates, H.C.; Verboket, R.D.; et al. Emerging multianalyte biosensors for the simultaneous detection of protein and nucleic acid biomarkers. Biosens. Bioelectron. 2024, 244, 115800.
- 19.Chen, Q.; Yao, L.; Wu, Q.; et al. Rapid and simultaneous visual typing of high-risk HPV-16/18 with use of integrated lateral flow strip platform. Microchim. Acta 2022, 189, 350.
- 20.Miller, B.S.; Bezinge, L.; Gliddon, H.D.; et al. Spin-enhanced nanodiamond biosensing for ultrasensitive diagnostics. Nature 2020, 587, 588–593.
- 21.Tran, V.; Walkenfort, B.; König, M.; et al. Rapid, quantitative, and ultrasensitive point-of-care testing: A portable SERS reader for lateral flow assays in clinical chemistry. Angew. Chem. Int. Edgl. 2019, 58, 442–446.
- 22.Ding, X.; Ma, J.; Fan, T.; et al. Inorganic nanoparticles-based strategies for the microbial detection in infectious diseases. Interdiscip. Med. 2024, 2, e20230045.
- 23.Wei, R.; Wang, D.; Zhou, P.; et al. A lateral flow assay strip for simultaneous detection of miRNA and exosomes in liver cancer. Chem. Commun. 2024, 60, 7491–7494.
- 24.Wang, Z.; Zhao, J.; Xu, X.; et al. An overview for the nanoparticles-based quantitative lateral flow assay. Small Methods 2022, 6, 2101143.
- 25.Su, L.H.; Chen, Y.Q.; Wang, L.L.; et al. Dual-signal based immunoassay for colorimetric and photothermal detection of furazolidone. Sensor Actuat. B-Chem. 2021, 331, 129431.
- 26.Sena-Torralba, A.; Torné-Morató, H.; Parolo, C.; et al. A novel ratiometric fluorescent approach for the modulation of the dynamic range of lateral flow immunoassays. Adv. Mater. Technol.-US 2022, 7, 2101450.
- 27.Wu, G.; Du, C.; Peng, C.; et al. Machine learning-assisted laccase-like activity nanozyme for intelligently onsite real-time and dynamic analysis of pyrethroid pesticides. J. Hazard. Mater. 2024, 480, 136015.
- 28.Liu, X.; Chen, X.; Yin, S.; et al. Dual-modal detection of antimicrobial susceptibility in pathogenic bacteria based on the high-throughput microfluidic platform. Chem. Eng. J. 2024, 499, 156506.
- 29.Cao, L.; Ren, Y.; Ling, N.; et al. An ultrasensitive smartphone-assisted bicolor-ratiometric fluorescence sensing platform based on a “noise purifier” for point-of-care testing of pathogenic bacteria in food. Food Chem. 2024, 446, 138805.
- 30.Wang, S.; Liang, N.; Hu, X.; et al. Carbon dots and covalent organic frameworks based FRET immunosensor for sensitive detection of Escherichia coli O157:H7. Food Chem. 2024, 447, 138663.
- 31.Koo, J.J.; Jung, K.H.; Park, K.; et al. Characterization of the Interfacial Structures of Core/Shell CdSe/ZnS QDs. J. Phys. Chem. Lett. 2022, 13, 7220–7227.
- 32.Nie, Y.; Liu, Y.; Zhang, Q.; et al. Fe3O4 NP@ZIF-8/MoS2 QD-based electrochemiluminescence with nanosurface energy transfer strategy for point-of-care determination of ATP. Anal. Chim. Acta 2020, 1127, 190–197.
- 33.Lao, X.; Liu, Y.; Li, L.; et al. Plasmon-enhanced FRET biosensor based on Tm3+/Er3+ co-doped core-shell upconversion nanoparticles for ultrasensitive virus detection. Aggregate 2024, 5, e448.
- 34.Castro, R.C.; Pascoa, R.; Saraiva, M.; et al. Exploring Distinct Second-Order Data Approaches for Thiamine Quantification via Carbon Dot/Silver Nanoparticle FRET Reversion. Biosensors 2024, 14, 604.
- 35.Wang, Z.; Xing, K.; Ding, N.; et al. Lateral flow immunoassay based on dual spectral-overlapped fluorescence quenching of polydopamine nanospheres for sensitive detection of sulfamethazine. J. Hazard. Mater. 2022, 423, 127204.
- 36.Shan, X.; Lu, J.; Li, C.; et al. Ultrasensitive solid-state electrochemiluminescence sensor based on lotus root shaped carbon fiber, CdSe QDs and Fe3O4 synergically amplify Ru (bpy) 32+ luminophore signal for detection of cyfluthrin. Microchim. Acta 2024, 191, 215.
- 37.Wang, S.; Zong, Z.; Xu, J.; et al. Recognition-Activated Primer-Mediated Exponential Rolling Circle Amplification for Signal Probe Production and Ultrasensitive Visual Detection of Ochratoxin A with Nucleic Acid Lateral Flow Strips. Anal. Chem. 2023, 95, 16398–16406.
Issue
Volume 1, Issue 1How to Cite
Chen, Q.; Yao, L.; Qu, W.; Xu, H.; Yan, C.; Xu, J.; Chen, W. Dual-Mode Lateral Flow Strip with Förster Resonance Energy Transfer for Rapid and Accurate Thrombin Detection. Bioelectrochemical Systems and Applications 2025, 1 (1), 2.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


