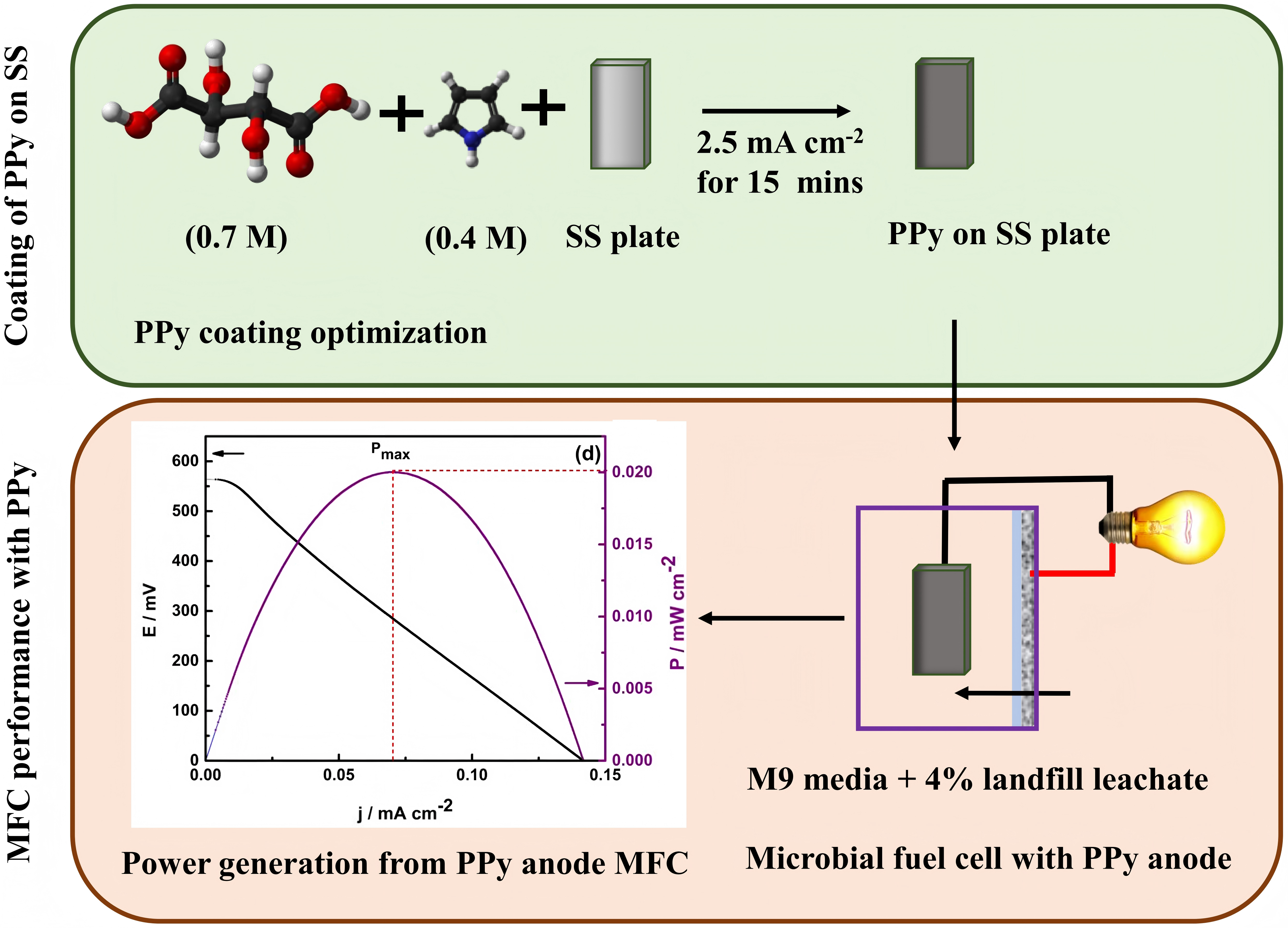
The use of stainless steel (SS) as an anode in a microbial fuel cell (MFC) suffers from significant limitations, such as poor biocompatibility, high activation overpotential, high charge transfer resistance, poor corrosion resistance, and its hydrophobic nature can hinder electron transfer. In this study, we present a robust approach for producing a low-cost, durable and biocompatible polyprrole (PPy) coating on a stainless plate (SS-P) anode for high performance MFC in harsh environments. The established galvanostatic polymerisation conditions produced PPy/SS-P anodes with improved surface area, enhanced electron transfer and good biocompatibility for enhanced MFC performance. Optimum PPy coating on the SS-P was achieved with an applied constant current density of 2.5 mA cm−2 for 15 min in a 0.7 M L-(+)-Tartaric acid solution, which contained 0.4 M Py. The effective formation of the PPy film on the SS-P was confirmed by chronopotentiometry and Fourier transform infrared (FTIR) spectroscopy. The nature of the PPy coating and its degree of hydrophilicity were investigated by contact angle measurements. It was found, for the first time, that this coating can transition from hydrophobic to hydrophilic upon exposure to an aqueous solution. This had a significant influence on the integrity and performance of the PPy coating when utilised for MFC. Also, in-depth analytical characterization of the PPy/SS-P was conducted by 3D profilometry and time-dependent electrochemical spectroscopy to provide insights into the nature and durability of the coatings. The subsequent utilization of the PPy/SS-P anodes in a single-chamber MFC gave a much lower open circuit voltage (OCVmax) of 355 ± 33 mV and 624 ± 47 mV for the first and second cycles, compared to 608 ± 32 mV and 664 ± 27 mV obtained for the SS-P anode. Also, the jmax of 0.027 ± 0.002 mA cm−2 and a Pmax of 0.020 ± 0.009 mW cm−2 obtained with the PPy/SS-P anode were much higher than with the SS-P anode, which gave jmax of 0.0012 ± 0.0008 mA cm−2 and Pmax of 0.010 ± 0.003 mW cm−2. Furthermore, a 3-fold increase in electricity production was achieved during the startup phase with the PPy/SS-P anode, but such an increase was not realised with the SS-P anode. The proposed PPy/SS-P anode, therefore, offers a great promise for use as a low-cost anode in MFC.
- Open Access
- Article
Fabrication of A Robust Low-Cost High Performance Stainless Steel-Based Polypyrrole Anode for Microbial Fuel Cells
- Jayesh M. Sonawane 1,*,
- Isha Kohli 2,
- R. K. Singh Raman 2,
- Prakash C. Ghosh 3,
- Samuel Adeloju 4,*
Author Information
Received: 02 Feb 2025 | Revised: 01 Sep 2025 | Accepted: 25 Sep 2025 | Published: 01 Oct 2025
Abstract
Graphical Abstract
Keywords
stainless steel | polypyrrole | galvanostatic polymerisation | high-performance anode | biomass growth | microbial fuel cell
References
- 1.Sonawane, J.M.; Yadav, A.; Ghosh, P.C.; et al. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 2017, 90, 558–576. https://doi.org/10.1016/j.bios.2016.10.014.
- 2.Santoro, C.; Arbizzani, C.; Erable, B.; et al. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. https://doi.org/10.1016/j.jpowsour.2017.03.109.
- 3.Sonawane, J.M.; Goenka, R.; Ghosh, P.C.; et al. Electrifying Waste Management: Integration of Polyaniline-Coated Electrodes in a Microbial Fuel Cell Stack for Power Generation and Leachate Treatment. Environ. Sci. Technol. 2023, 57, 6250–6260. https://doi.org/10.2139/SSRN.4600892.
- 4.Ucar, D.; Zhang, Y.; Angelidaki, I. An overview of electron acceptors in microbial fuel cells. Front. Microbiol. 2017, 8, 643. https://doi.org/10.3389/fmicb.2017.00643.
- 5.Hirooka, K.; Ichihashi, O.; Takeguchi, T. Sodium cobalt oxide as a non-platinum cathode catalyst for microbial fuel cells. Sustain. Environ. Res. 2018, 28, 322–325. https://doi.org/10.1016/j.serj.2018.07.002.
- 6.Baudler, A.; Schmidt, I.; Langner, M.; et al. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. https://doi.org/10.1039/C5EE00866B.
- 7.Yuan, H.; He, Z. Graphene-modified electrodes for enhancing the performance of microbial fuel cells. Nanoscale 2015, 7, 7022–7029. https://doi.org/10.1039/c4nr05637j.
- 8.Peng, X.H.; Chu, X.Z.; Huang, P.F.; et al. Improved Power Performance of Activated Carbon Anode by Fe2O3 Addition in Microbial Fuel Cells. Appl. Mech. Mater. 2014, 700, 170–174. https://doi.org/10.4028/www.scientific.net/amm.700.170.
- 9.Wu, G.; Bao, H.; Xia, Z.; et al. Polypyrrole/sargassum activated carbon modified stainless-steel sponge as high-performance and low-cost bioanode for microbial fuel cells. J. Power Sources 2018, 384, 86–92. https://doi.org/10.1016/j.jpowsour.2018.02.045.
- 10.Mustakeem. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 22. https://doi.org/10.1007/s40243-015-0063-8.
- 11.Zhu, X.; Logan, B.E. Copper anode corrosion affects power generation in microbial fuel cells. J. Chem. Technol. Biotechnol. 2014, 89, 471–474.
- 12.Peng, X.; Chen, S.; Liu, L.; et al. Modified stainless steel for high performance and stable anode in microbial fuel cells. Electrochim. Acta 2016, 194, 246–252. https://doi.org/10.1016/j.electacta.2016.02.127.
- 13.He, Y.-R.; Xiao, X.; Li, W.-W.; et al. Enhanced electricity production from microbial fuel cells with plasma-modified carbon paper anode. Phys. Chem. Chem. Phys. 2012, 14, 9966–9971. https://doi.org/10.1039/c2cp40873b.
- 14.Mahadevan, A.; Gunawardena, D.A.; Fernando, S. Technology and Application of Microbial Fuel Cells. In Technology and Application of Microbial Fuel Cells; Bentham Science Publishers: Oak Park, IL, USA, 2014; pp. 13–32. https://doi.org/ 10.5772/57200.
- 15.e Silva, T.C.A.; Bhowmick, G.D.; Ghangrekar, M.M.; et al. SiOC-based polymer derived-ceramic porous anodes for microbial fuel cells. Biochem. Eng. J. 2019, 148, 29–36.
- 16.Cui, H.F.; Du, L.; Guo, P.B.; et al. Controlled modification of carbon nanotubes and polyaniline on macroporous graphite felt for high-performance microbial fuel cell anode. J. Power Source 2015, 283, 46–53.
- 17.Nosek, D.; Jachimowicz, P.; Cydzik-Kwiatkowska, A. Anode modification as an alternative approach to improve electricity generation in microbial fuel cells. Energies 2020, 13, 6596.
- 18.Kovendhan, M.; Kang, H.; Jeong, S.; Youn, J-S.; Oh, Park, Y-K.; Jeon, K-J. Study of stainless steel electrodes after electrochemical analysis in sea water condition, Environmental Research 2019, 173, 549–555.
- 19.Hou, J.; Liu, Z.; Yang, S.; et al. Three-dimensional macroporous anodes based on stainless steel fiber felt for high-performance microbial fuel cells. J. Power Sources 2014, 258, 204–209. https://doi.org/10.1016/j.jpowsour.2014.02.035.
- 20.Pocaznoi, D.; Calmet, A.; Etcheverry, L.; et al. Stainless steel is a promising electrode material for anodes of microbial fuel cells. Energy Environ. Sci. 2012, 5, 9645–9652. https://doi.org/10.1039/C2EE22429A.
- 21.Hou, J.; Liu, Z.; Li, Y. Polyaniline Modified Stainless Steel Fiber Felt for High-Performance Microbial Fuel Cell Anodes. J. Clean Energy Technol. 2015, 3, 165–169. https://doi.org/10.7763/JOCET.2015.V3.189.
- 22.Song, R.B.; Wu, Y.C.; Lin, Z.Q.; et al. Living and Conducting: Coating Individual Bacterial Cells with In Situ Formed Polypyrrole. Angew. Chem. Int. Ed. Engl. 2017, 56, 10516–10520. https://doi.org/10.1002/anie.201704729.
- 23.Yuan, Y.; Kim, S. Polypyrrole-coated reticulated vitreous carbon as anode in microbial fuel cell for higher energy output. Bull. Korean Chem. Soc. 2008, 29, 168–172. https://doi.org/10.5012/bkcs.2008.29.1.168.
- 24.Tang, X.; Li, H.; Du, Z.; et al. Conductive polypyrrole hydrogels and carbon nanotubes composite as an anode for microbial fuel cells. RSC Adv. 2015, 5, 50968–50974. https://doi.org/10.1039/C5RA06064H.
- 25.Kamali, S.; Esfandyari, M.; Jafari, D. A review of the application of polymeric materials in microbial fuel cells. Polym. Bull. 2025, 82:7465–7492.
- 26.Lin, X.Q.; Li, Z.L.; Liang, B.; et al. Identification of biofilm formation and exoelectrogenic population structure and function with graphene/polyanliline modified anode in microbial fuel cell. Chemosphere 2019, 219, 358–364.
- 27.Zhang, P.; Zhou, X.; Qi, R.; et al. Conductive polymer–exoelectrogen hybrid bioelectrode with improved biofilm formation and extracellular electron transport. Adv. Electron. Mater. 2019, 5, 1900320.
- 28.Kang, Y.L.; Pichiah, S.; Ibrahim, S. Facile reconstruction of microbial fuel cell (MFC) anode with enhanced exoelectrogens selection for intensified electricity generation. Int. J. Hydrog. Energy 2017, 42, 1661–1671.
- 29.Kumar, A.; Narayanan, S.S.; Thapa, B.S.; et al. Application of low-cost plant-derived carbon dots as a sustainable anode catalyst in microbial fuel cells for improved wastewater treatment and power output. Catalysts 2022, 12, 1580.
- 30.Sonawane, J.M.; Al-Saadi, S.; Raman, R.K.S.; et al. Exploring the use of polyaniline-modified stainless steel plates as low-cost, high-performance anodes for microbial fuel cells. Electrochim. Acta 2018, 268, 484–493. https://doi.org/10.1016/j.electacta. 2018. 01.163.
- 31.Sonawane, J.M.; Patil, S.A.; Ghosh, P.C.; et al. Low-cost stainless-steel wool anodes modified with polyaniline and polypyrrole for high-performance microbial fuel cells. J. Power Sources 2018, 379, 103–114. https://doi.org/10.1016/j.jpowsour.2018.01.001.
- 32.Pu, K.B.; Ma, Q.; Cai, W.F.; et al. Polypyrrole modified stainless steel as high performance anode of microbial fuel cell. Biochem. Eng. J. 2018, 132, 255–261.
- 33.Sonawane, J.M.; Ghosh, P.C.; Adeloju, S.B. Electrokinetic behaviour of conducting polymer modified stainless steel anodes during the enrichment phase in microbial fuel cells. Electrochim. Acta 2018, 287, 96–105. https://doi.org/10.1016/j.electacta.2018.07.077.
- 34.Gajda, I.; Greenman, J.; Ieropoulos, I.A. Recent advancements in real-world microbial fuel cell applications. Curr. Opin. Electrochem. 2018, 11, 78–83. https://doi.org/10.1016/j.coelec.2018.09.006.
- 35.Gnana Kumar, G.; Kirubaharan, C.J.; Udhayakumar, S.; et al. Synthesis, structural, and morphological characterizations of reduced graphene oxide-supported polypyrrole anode catalysts for improved microbial fuel cell performances. ACS Sustain. Chem. Eng. 2014, 2, 2283–2290. https://doi.org/10.1021/sc500244f.
- 36.Pérez-Rodríguez, P.; Ovando-Medina, V.M.; Martínez-Amador, S.Y.; et al. Bioanode of polyurethane/graphite/polypyrrole composite in microbial fuel cells. Biotechnol. Bioprocess Eng. 2016, 21, 305–313. https://doi.org/10.1007/s12257-015-0628-5.
- 37.Chen, W.; Liu, Z.; Su, G.; et al. Composite-modified anode by MnO 2 /polypyrrole in marine benthic microbial fuel cells and its electrochemical performance. Int. J. Energy Res. 2017, 41, 845–853. https://doi.org/10.1002/er.3674.
- 38.Roh, S.-H. Electricity Generation from Microbial Fuel Cell with Polypyrrole-Coated Carbon Nanofiber Composite. J. Nanosci. Nanotechnol. 2015, 15, 1700–1703.
- 39.Zou, Y.; Xiang, C.; Yang, L.; et al. A mediatorless microbial fuel cell using polypyrrole coated carbon nanotubes composite as anode material. Int. J. Hydrog. Energy 2008, 33, 4856–4862. https://doi.org/10.1016/j.ijhydene.2008.06.061.
- 40.Harnisch, F.; Koch, C.; Patil, S.A.; et al. Revealing the electrochemically driven selection in natural community derived microbial biofilms using flow-cytometry. Energy Environ. Sci. 2011, 4, 1265–1267. https://doi.org/10.1039/c0ee00605j.
- 41.Liu, Y.; Harnisch, F.; Fricke, K.; et al. Improvement of the anodic bioelectrocatalytic activity of mixed culture biofilms by a simple consecutive electrochemical selection procedure. Biosens. Bioelectron. 2008, 24, 1006–1011. https://doi.org/10.1016/j.bios.2008.08.001.
- 42.Sonawane, J.M.; Adeloju, S.B.; Ghosh, P.C. Landfill leachate: A promising substrate for microbial fuel cells. Int. J. Hydrog. Energy 2017, 42, 23794–23798. https://doi.org/10.1016/j.ijhydene.2017.03.137.
- 43.Ramoa, S.D.A.; Barra, G.M.O.; Merlini, C.; et al. Novel electrically conductive polyurethane/montmorillonite-polypyrrole nanocomposites. Express Polym. Lett. 2015, 9, 945–958.
- 44.Raotole, P.; Patil, P.P.; Gaikwad, A.B. Polypyrrole Coatings on Low Carbon Steel from Aqueous Oxalate Solution. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 62–67.
- 45.Vetter, C.A.; Gelling, V.J. Template-Free Aqueous Synthesis of Conductive Polymer Nanoparticles. US20140110636A1, 24 April 2014.
- 46.Li, Z.; Cai, J.; Cizek, P.; et al. A self-supported, flexible, binder-free pseudo-supercapacitor electrode material with high capacitance and cycling stability from hollow, capsular polypyrrole fibers. J. Mater. Chem. A 2015, 3, 16162–16167.
- 47.Chang, J.H.; Hunter, I.W. Characterization and control of the wettability of conducting polymer thin films. In Proceedings of the Materials Research Society Symposium, 31 January 2011; pp. 7–12. https://doi.org/10.1557/PROC-1228-KK04-03.
- 48.Thombare, J.V.; Lohar, G.M.; Shinde, S.K.; et al. Synthesis, characterization and surface wettability study of polypyrrole films: Effect of applied constant current density. Electron. Mater. Lett. 2015, 11, 266–270. https://doi.org/10.1007/s13391-014-4082-x.
- 49.Valtera, S.; Prokeš, J.; Kopecká, J.; et al. Dye-stimulated control of conducting polypyrrole morphology. RSC Adv. 2017, 7, 51495–51505. https://doi.org/10.1039/c7ra10027b.
- 50.Shen, X.; Xu, X.; Li, C.; et al. Robust coating for high-temperature and corrosion-resistant. J. Vac. Sci. Technol. A 2024, 42. https://doi.org/10.1116/6.0003954.
- 51.Goldoni, R.; Thomaz, D.V.; Ottolini, M.; et al. Characterization of In situ electrosynthesis of polyaniline on pencil graphite electrodes through electrochemical, spectroscopical and computational methods. J. Mater. Sci. 2024, 59, 10287–10308. https://doi.org/10.1007/S10853-024-09745-8.
- 52.Ha, P.T.; Moon, H.; Kim, B.H.; et al. Determination of charge transfer resistance and capacitance of microbial fuel cell through a transient response analysis of cell voltage. Biosens.Bioelectron. 2010, 25, 1629–1634.
- 53.Altahan, M.F.; Beltagi, A.M.; Abdel-Azzem, M.; et al. An impedimetric approach for determination of ammonium using silver/poly-1-aminoanthraquinone/carbon paste electrode. Sci Rep. 2024, 14, 18555. https://doi.org/10.1038/s41598-024-68321-x].
- 54.Ramesh, D. Evaluation of Corrosion Stability of Water Soluble Epoxy-Ester Primer through Electrochemical Studies. Mater. Sci. Appl. 2012, 3, 333–347.
- 55.Fan, C.; Liu, Y.; Yin, X.; et al. Electrochemical Behavior and Interfacial Delamination of a Polymer-Coated Galvanized Steel System in Acid Media. ACS Omega 2021, 6, 20331–20340. https://doi.org/10.1021/acsomega. 1c02270.
- 56.Zuo, Y.; Pang, R.; Li, W.; et al. The evaluation of coating performance by the variations of phase angles in middle and high frequency domains of EIS. Corros. Sci. 2008, 50, 3322–3328.
- 57.Yuan, H.; Deng, L.; Chen, Y.; et al. MnO2/Polypyrrole/MnO2 multi-walled-nanotube-modified anode for high-performance microbial fuel cells. Electrochim. Acta 2016, 196, 280–285. https://doi.org/10.1016/j.electacta.2016.02.183.
Issue
Volume 1, Issue 1How to Cite
Sonawane, J. M.; Kohli, I.; Raman, R. K. S.; Ghosh, P. C.; Adeloju, S. Fabrication of A Robust Low-Cost High Performance Stainless Steel-Based Polypyrrole Anode for Microbial Fuel Cells. Bioelectrochemical Systems and Applications 2025, 1 (1), 3.
RIS
BibTex
Copyright & License
Contents
References


