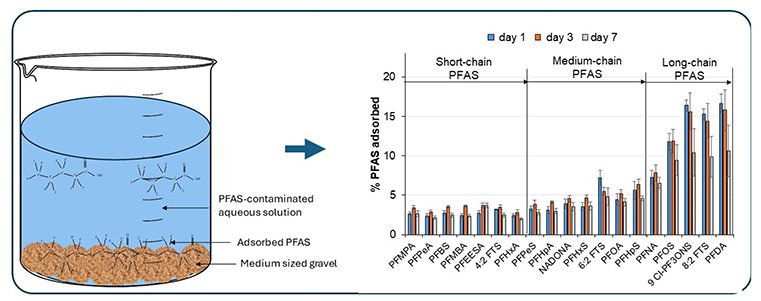
Poly- and perfluoroalkyl substances (PFAS) are persistent chemicals that may pose risks to ecosystems and human health. Understanding the environmental fate and transport of PFAS is challenging due to their ability to migrate across air, water, and soil. In surface waters, PFAS can interact with sediments, organic matter, and plants, influencing the mobility of these compounds and posing potential risks to the environment. This study provides the first analysis of the adsorption of PFAS, including perfluoro-carboxylic acids (PFCA, C4–C10), perfluoro-sulfonic acids (PFSA, C4–C8), per-/poly-fluoroalkylether acids (PFEA C4-C8) and fluorotelomer sulfonates (FTS, C8 and C10) to medium-sized quartz gravel (pebbles), commonly found in the UK river systems. The effects of exposure time (1, 3 and 7 days) and mechanical disturbance (shaking) on PFAS adsorption were evaluated. The degree of PFAS adsorption indicated a clear dependence on the compound’s functional group and carbon chain length. Long-chain PFAS, perfluorodecanoic acid (PFDA), exhibited the highest adsorption, while PFCA showed the least sorption compared to corresponding PFEA, PFSA and FTS of homologues (C5–C8). Mechanical disturbance (shaking) of gravel in PFAS-contaminated water did not significantly influence the extent of adsorption on most of the studied analytes except 9 Cl-PF3OUdS, 8:2 FTS, and PFDA, onto the gravel. The study demonstrates, for the first time, that medium-sized quartz gravel can adsorb PFAS, including new-generation substitutes from water. In riverine systems, these pollutants can be remobilised from gravel surfaces during e.g., flooding, dredging, or changes in water chemistry, potentially reintroducing them into the water and impacting water quality and ecosystem.
- Open Access
- Article
Investigation of Per- and Polyfluoroalkyl Substances (PFAS) Adsorption onto the Medium Size Quartz Gravel
- Omotola Folorunsho,
- Anna Bogush,
- Ivan Kourtchev *
Author Information
Received: 14 Jun 2025 | Revised: 14 Jul 2025 | Accepted: 11 Aug 2025 | Published: 22 Aug 2025
Abstract
Graphical Abstract
Keywords
quartz | PFOS | PFOA | PFHxA | water pollution
References
- 1.Glüge, J.; Scheringer, M.; Cousins, I.T.; et al. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process Impacts 2020, 22, 2345–2373. https://doi.org/10.1039/D0EM00291G.
- 2.Fenton, S.E.; Ducatman, A.; Boobis, A.; et al. Per-and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. https://doi.org/10.1002/etc.4890
- 3.Banyoi, S.M.; Porseryd, T.; Larsson, J.; et al. The effects of exposure to environmentally relevant PFAS concentrations for aquatic organisms at different consumer trophic levels: Systematic review and meta-analyses. Environ. Pollut. 2022, 315, 120422. https://doi.org/10.1016/J.ENVPOL.2022.120422.
- 4.Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; et al. Scientific basis for managing PFAS as a chemical class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. https://doi.org/10.1021/acs.estlett.0c00255.
- 5.US EPA. PFAS Structures in DSSTox. 2022. Available online: https://comptox.epa.gov/dashboard/chemical-lists/PFASSTRUCTV5 (accessed on 30 January 2025).
- 6.Ackerman Grunfeld, D.; Gilbert, D.; Hou, J.; et al. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024, 17, 340–346. https://doi.org/10.1038/s41561-024-01402-8.
- 7.Stockholm Convention. 2024. All POPs Listed in the Stockholm Convention. Available online: https://www.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 30 January 2025).
- 8.UK Drinking Water Inspectorate (UKDWI). Guidance on the Water Supply (Water Quality) Regulations 2016 (as Amended) for England and Water Supply (Water Quality) Regulations 2018 for Wales Specific to PFAS (per- and Polyfluoroalkyl Substances) in Drinking Water. 2025. Available online: https://dwi-production-files.s3.eu-west-2.amazonaws.com/wp-content/uploads/2025/03/24141825/DWI_PFAS-Guidance_Mar_2025.pdf (accessed on 7 July 2025).
- 9.UK Drinking Water Inspectorate (UKDWI). Guidance on the Water Supply (Water Quality) Regulations 2016 Specific to PFOS (Perfluorooctane Sulphonate) and PFOA (Perfluorooctanoic Acid) Concentrations in Drinking Water. 2021. Available online: https://cdn.dwi.gov.uk/wp-content/uploads/2021/01/12110137/PFOS-PFOA-guidance-2021.pdf (accessed on 7 July 2025).
- 10.Gago-Ferrero, P.; Gros, M.; Ahrens, L.; et al. Impact of on-site, small and large scale wastewater treatment facilities on levels and fate of pharmaceuticals, personal care products, artificial sweeteners, pesticides, and perfluoroalkyl substances in recipient waters. Sci. Total Environ. 2017, 601–602, 1289–1297. https://doi.org/10.1016/J.SCITOTENV.2017.05.258.
- 11.Ciofi, L.; Renai, L.; Rossini, D.; et al. Applicability of the direct injection liquid chromatographic tandem mass spectrometric analytical approach to the sub-ng L−1 determination of perfluoro-alkyl acids in waste, surface, ground and drinking water samples. Talanta 2018, 176, 412–421. https://doi.org/10.1016/J.TALANTA.2017.08.052.
- 12.Folorunsho, O.; Bogush, A.; Kourtchev, I. A new on-line SPE LC-HRMS method for simultaneous analysis of selected emerging contaminants in surface waters. Anal. Methods 2023, 15, 284–296. https://doi.org/10.1039/D2AY01574A.
- 13.Sharma, B.M.; Bharat, G.K.; Tayal, S.; et al. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human exposure. Environ. Pollut. 2016, 208, 704–713. https://doi.org/10.1016/J.ENVPOL.2015.10.050.
- 14.Crone, B.C.; Speth, T.F.; Wahman, D.G.; et al. Occurrence of per- and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. https://doi.org/10.1080/10643389.2019.1614848.
- 15.Wong, F.; Hung, H.; Dryfhout-Clark, H.; et al. Time trends of persistent organic pollutants (POPs) and Chemicals of Emerging Arctic Concern (CEAC) in Arctic air from 25 years of monitoring. Sci. Total Environ. 2021, 775, 145109. https://doi.org/10.1016/J.SCITOTENV.2021.145109.
- 16.Zhou, J.; Baumann, K.; Mead, R.N.; et al. PFOS dominates PFAS composition in ambient fine particulate matter (PM2.5) collected across North Carolina nearly 20 years after the end of its US production. Environ. Sci. Process Impacts 2021, 23, 580–587. https://doi.org/10.1039/d0em00497a.
- 17.Kourtchev, I.; Hellebust, S.; Heffernan, E.; et al. A new on-line SPE LC-HRMS method for the analysis of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in PM2.5 and its application for screening atmospheric particulates from Dublin and Enniscorthy, Ireland. Sci. Total Environ. 2022, 835, 155496. https://doi.org/10.1016/J.SCITOTENV.2022.155496.
- 18.Qi, Y.; Huo, S.; Xi, B.; et al. Spatial distribution and source apportionment of PFASs in surface sediments from five lake regions, China. Sci. Rep. 2016, 6, 22674. https://doi.org/10.1038/srep22674.
- 19.Ahmadireskety, A.; Da Silva, B.F.; Townsend, T.G.; et al. Evaluation of extraction workflows for quantitative analysis of per- and polyfluoroalkyl substances: A case study using soil adjacent to a landfill. Sci. Total Environ. 2021, 760, 143944. https://doi.org/10.1016/J.SCITOTENV.2020.143944.
- 20.Chirikona, F.; Quinete, N.; Gonzalez, J.; Mutua, G.; et al. Occurrence and distribution of per- and polyfluoroalkyl substances from multi-industry sources to water, sediments and plants along Nairobi River basin, Kenya. Int. J. Environ. Res. Public Health 2022, 19, 8980. https://doi.org/10.3390/ijerph19158980.
- 21.Pfotenhauer, D.; Sellers, E.; Olson, M.; et al. PFAS concentrations and deposition in precipitation: An intensive 5-month study at national atmospheric deposition program—National trends sites (NADP-NTN) across Wisconsin, USA. Atmos. Environ. 2022, 291, 119368. https://doi.org/10.1016/J.ATMOSENV.2022.119368.
- 22.Hamid, H.; Li, L.Y.; Grace, J.R. Review of the fate and transformation of per- and polyfluoroalkyl substances (PFASs) in landfills. Environ. Pollut. 2018, 235, 74–84. https://doi.org/10.1016/J.ENVPOL.2017.12.030.
- 23.Zhang, M.; Zhao, X.; Zhao, D.; et al. Poly- and perfluoroalkyl substances (PFAS) in landfills: Occurrence, transformation and treatment. J. Waste Manag. 2023, 155, 162–178. https://doi.org/10.1016/J.WASMAN.2022.10.028.
- 24.Houtz, E.F.; Sutton, R.; Park, J.S.; et al. Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res. 2016, 95, 142–149. https://doi.org/10.1016/J.WATRES.2016.02.055.
- 25.Kurwadkar, S.; Dane, J.; Kanel, S.R.; et al. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. https://doi.org/10.1016/J.SCITOTENV.2021.151003.
- 26.Borthakur, A.; Wang, M.; He, M.; et al. Perfluoroalkyl acids on suspended particles: Significant transport pathways in surface runoff, surface waters, and subsurface soils. J. Hazard. Mater. 2021, 417, 126159. https://doi.org/10.1016/J.JHAZMAT.2021.126159.
- 27.Balgooyen, S.; Remucal, C.K. Tributary loading and sediment desorption as sources of pfas to receiving waters. ACS ES&T Water 2022, 2, 436–445. https://doi.org/10.1021/acsestwater.1c00348.
- 28.Zhao, Y.; Min, X.; Xu, S.; et al. Adsorption of per- and polyfluoroalkyl substances (PFAS) by aquifer materials: The important role of dolomite. Environ. Sci. Technol. Lett. 2023, 10, 931–936. https://doi.org/10.1021/acs.estlett.3c00583.
- 29.Harfmann, J.L.; Tito, K.; Kieber, R.J.; et al. Sorption of hexafluoropropylene oxide dimer acid to sediments: Biogeochemical implications and analytical considerations. ACS Earth Space Chem. 2021, 5, 580–587. https://doi.org/10.1021/acsearthspacechem.0c00323.
- 30.Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S.; et al. Sorption and partitioning of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) onto sediments of Diep and Plankenburg river systems Western Cape, South Africa. Environ. Technol. Innov. 2022, 25, 102110. https://doi.org/10.1016/J.ETI.2021.102110.
- 31.Reif, D.; Zoboli, O.; Wolfram, G.; et al. Pollutant source or sink? Adsorption and mobilization of PFOS and PFOA from sediments in a large shallow lake with extended reed belt. J. Environ. Manag. 2022, 320, 115871. https://doi.org/10.1016/J.JENVMAN.2022.115871.
- 32.Milinovic, J.; Lacorte, S.; Vidal, M.; et al. Sorption behaviour of perfluoroalkyl substances in soils. Sci. Total Environ. 2015, 511, 63–71. https://doi.org/10.1016/j.scitotenv.2014.12.017.
- 33.Miao, Y.; Guo, X.; Dan Peng Fan, T.; et al. Rates and equilibria of perfluorooctanoate (PFOA) sorption on soils from different regions of China. Ecotoxicol. Environ. Saf. 2017, 139, 102–108. https://doi.org/10.1016/J.ECOENV.2017.01.022.
- 34.Nguyen, T.M.H.; Bräunig, J.; Thompson, K.; et al. Influences of chemical properties, soil properties, and solution pH on soil-water partitioning coefficients of per- and polyfluoroalkyl substances (PFASs). Environ. Sci. Technol. 2020, 54, 15883–15892.
- 35.Oliver, D.P.; Li, Y.; Orr, R.; et al. Sorption behaviour of per- and polyfluoroalkyl substances (PFASs) in tropical soils. Environ. Pollut. 2020, 258, 113726. https://doi.org/10.1016/J.ENVPOL.2019.113726.
- 36.Son, H.; Kim, T.; Yoom, H.S.; et al. The adsorption selectivity of short and long per-and polyfluoroalkyl substances (PFASs) from surface water using powder-activated carbon. Water 2020, 12, 3287. https://doi.org/10.3390/w12113287.
- 37.Niarchos, G.; Georgii, L.; Ahrens, L.; et al. A systematic study of the competitive sorption of per- and polyfluoroalkyl substances (PFAS) on colloidal activated carbon. Ecotoxicol Environ Saf 2023, 264, 115408. https://doi.org/10.1016/J.ECOENV.2023.115408.
- 38.Lyu, X.; Xiao, F.; Shen, C.; et al. Per- and polyfluoroalkyl substances (PFAS) in subsurface environments: Occurrence, fate, transport, and research prospect. Rev. Geophys. 2022, 60, e2021RG000765. https://doi.org/10.1029/2021RG000765.
- 39.Johnson, R.L.; Anschutz, A.J.; Smolen, J.M.; et al. The adsorption of perfluorooctane sulfonate onto sand, clay, and iron oxide surfaces. J. Chem. Eng. Data 2007, 52, 1165–1170. https://doi.org/10.1021/je060285g.
- 40.Hellsing, M.S.; Josefsson, S.; Hughes, A.V.; et al. Sorption of perfluoroalkyl substances to two types of minerals. Chemosphere 2016, 159, 385–391. https://doi.org/10.1016/J.CHEMOSPHERE.2016.06.016.
- 41.Hubert, M.; Arp, H.P.H.; Hansen, M.C.; et al. Influence of grain size, organic carbon and organic matter residue content on the sorption of per- and polyfluoroalkyl substances in aqueous film forming foam contaminated soils–Implications for remediation using soil washing. Sci. Total Environ. 2023, 875, 162668. https://doi.org/10.1016/J.SCITOTENV.2023.162668
- 42.Higgins, C.P.; Luthy, R.G. Sorption of perfluorinated surfactants on sediments. Environ. Sci. Technol. 2006, 40, 7251–7256. https://doi.org/10.1021/es061000n.
- 43.Chen, H.; Reinhard, M.; Nguyen, V.T.; et al. Reversible and irreversible sorption of perfluorinated compounds (PFCs) by sediments of an urban reservoir. Chemosphere 2016, 144, 1747–1753. https://doi.org/10.1016/j.chemosphere.2015.10.055.
- 44.Du, Z.; Deng, S.; Bei, Y.; et al. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. https://doi.org/10.1016/J.JHAZMAT.2014.04.038
- 45.Sörengård, M.; Franke, V.; Tröger, R.; et al. Losses of poly- and perfluoroalkyl substances to syringe filter materials. J. Chromatogr. A 2020, 1609, 460430. https://doi.org/10.1016/J.CHROMA.2019.460430.
- 46.Brusseau, M.L. Differential sorption of short-chain versus long-chain anionic per- and poly-fluoroalkyl substances by soils. Environments 2023, 10, 175. https://doi.org/10.3390/environments10100175.
- 47.Owens, P.N.; Batalla, R.J.; Collins, A.J.; et al. Fine-grained sediment in river systems: Environmental significance and management issues. River Res. Appl. 2005, 21, 693–717. https://doi.org/10.1002/rra.878.
- 48.Neely, A.B.; DiBiase, R.A. Drainage Area, Bedrock Fracture Spacing, and Weathering Controls on Landscape-Scale Patterns in Surface Sediment Grain Size. J. Geophys. Res. Earth Surf. 2020, 125, e2020JF005560. https://doi.org/10.1029/2020JF005560.
- 49.Owens, P.N. Soil erosion and sediment dynamics in the Anthropocene: A review of human impacts during a period of rapid global environmental change. J. Soils Sediments 2020, 20, 4115–4143. https://doi.org/10.1007/s11368-020-02815-9/Published.
- 50.Cormier, B.; Borchet, F.; Kärrman, A.; et al. Sorption and desorption kinetics of PFOS to pristine microplastic. Environ. Sci. Pollut. Res. Int. 2022, 29, 4497–4507. https://doi.org/10.1007/s11356-021-15923-x/.
- 51.Fabregat-Palau, J.; Vidal, M.; Rigol, A. Modelling the sorption behaviour of perfluoroalkyl carboxylates and perfluoroalkane sulfonates in soils. Sci. Total Environ. 2021, 801, 149343. https://doi.org/10.1016/J.SCITOTENV.2021.149343.
- 52.Campos-Pereira, H.; Kleja, D.B.; Ahrens, L.; et al. Effect of pH, surface charge and soil properties on the solid–solution partitioning of perfluoroalkyl substances (PFASs) in a wide range of temperate soils. Chemosphere 2023, 321, 138133. https://doi.org/10.1016/J.CHEMOSPHERE.2023.138133.
- 53.Mcevoy, F.; Steadman, E.J.; Harrison, D.J.; et al. Yorkshire and the Humber Region: Sand and Gravel Resources and Environmental Assets. British Geological Survey Commissioned Report, CR/04/216N. 39p. 2004. Available online: https://nora.nerc.ac.uk/id/eprint/509795/1/CR04216N.pdf (accessed on 30 January 2025).
- 54.Zheng, P.; Liu, M.; Yin, H.; et al. Analysis of 58 poly-/perfluoroalkyl substances and their occurrence in surface water in a high-technology industrial park. Environ. Pollut. 2020, 267, 115381. https://doi.org/10.1016/J.ENVPOL.2020.115381.
- 55.Adeogun, A.O.; Chukwuka, A.V.; Ibor, O.R.; et al. Occurrence, bioaccumulation and trophic dynamics of per- and polyfluoroalkyl substances in two tropical freshwater lakes. Environ. Pollut. 2024, 346, 123575. https://doi.org/10.1016/J.ENVPOL.2024.123575.
- 56.Zarębska, M.; Bajkacz, S.; Hordyjewicz-Baran, Z. Assessment of legacy and emerging PFAS in the Oder River: Occurrence, distribution, and sources. Environ. Res. 2024, 251, 118608. https://doi.org/10.1016/J.ENVRES.2024.118608.
- 57.Lath, S.; Knight, E.R.; Navarro, D.A.; et al. Sorption of PFOA onto different laboratory materials: Filter membranes and centrifuge tubes. Chemosphere 2019, 222, 671–678. https://doi.org/10.1016/J.CHEMOSPHERE.2019.01.096.
- 58.Zenobio, J.E.; Salawu, O.A.; Han, Z.; et al. Adsorption of per- and polyfluoroalkyl substances (PFAS) to containers. J. Hazard Mater. Adv. 2022, 7, 100130. https://doi.org/10.1016/j.hazadv.2022.100130.
- 59.Folorunsho, O.; Kizhakkethil, J.P.; Bogush, A.; et al. Effect of short-term sample storage and preparatory conditions on losses of 18 per- and polyfluoroalkyl substances (PFAS) to container materials. Chemosphere 2024, 363, 142814. https://doi.org/10.1016/J.CHEMOSPHERE.2024.142814.
- 60.Morgan, C.J. Use of proper statistical techniques for research studies with small samples. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, 5. https://doi.org/10.1152/ajplung.00238.2017.
- 61.Vu, C.T.; Wu, T. Adsorption of short-chain perfluoroalkyl acids (PFAAs) from water/wastewater. Environ. Sci. 2020, 6, 2958–2972. https://doi.org/10.1039/d0ew00468e.
- 62.Helbling, D.E.; Dichtel, W.R. Final Report: Rational Design and Implementation of Novel Polymer Adsorbents for Selective Uptake of Pfas from Groundwater. SERDP Project, ER18-1026. 2022. Available online: https://apps.dtic.mil/sti/trecms/pdf/AD1187004.pdf (accessed on 30 January 2025).
- 63.Yin, Y.; Fan, C.; Cheng, L.; et al. Adsorption of perfluoroalkyl substances on deep eutectic solvent-based amorphous metal-organic framework: Structure and mechanism. Environ. Res. 2024, 248, 118261. https://doi.org/10.1016/J.ENVRES.2024.118261.
- 64.Zhang, R.; Yan, W.; Jing, C. Mechanistic study of PFOS adsorption on kaolinite and montmorillonite. Colloids Surf. A Physicochem. Eng. Asp. 2014, 462, 252–258. https://doi.org/10.1016/J.COLSURFA.2014.09.019.
- 65.Li, Y.; Oliver, D.P.; Kookana, R.S. A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2018, 628–629, 110–120. https://doi.org/10.1016/J.SCITOTENV.2018.01.167.
- 66.Zhao, Y.; Min, X.; Xu, S.; et al. Adsorption of per- and polyfluoroalkyl substances (PFAS) by aquifer materials: The important role of dolomite. Environ. Sci. Technol. Lett. 2023, 10, 931–936. https://doi.org/10.1021/acs.estlett.3c00583.
- 67.Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. https://doi.org/10.1016/J.CHEMOSPHERE.2022.136732.
- 68.Qadir, A.; Jamil, N.; Khan, S.M.; et al. Removal of direct red 16 (textile dye) from industrial effluent by using feldspar. J. Chem. Soc. Pak. 2014, 36, 191–197.
- 69.Kuśmierek, K.; Świątkowski, A. The influence of different agitation techniques on the adsorption kinetics of 4-chlorophenol on granular activated carbon. React. Kinet. Mech. Catal. 2015, 116, 261–271. https://doi.org/10.1007/s11144-015-0889-1.
How to Cite
Folorunsho, O.; Bogush, A.; Kourtchev, I. Investigation of Per- and Polyfluoroalkyl Substances (PFAS) Adsorption onto the Medium Size Quartz Gravel. Environmental Contamination: Causes and Solutions 2025, 1 (1), 4. https://doi.org/10.53941/eccs.2025.100004.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


