The supply of safe medical devices is of critical importance; however, many devices are increasing in complexity to reflect patient needs and for regulatory compliance. Single use devices (SUDs) have been extensively used in healthcare for various reasons including user convenience and perception of higher material quality, enhanced safety, and better mitigation of patient risk for device-associated infections. However, where appropriate, use of cleaned and processed medical devices are equally effective to that of using SUDs. Use of disposables has created considerable medical waste management issues globally. Consequently, this perspective review paper addresses key initiatives and recommendations for potentially improving a culture of medical device reuse and recycling in healthcare ranging from meeting scalability and predictability in supply chain to promoting green design thinking and regulation across micro, meso and macro levels of stakeholder engagement. Building such a comprehensive ecosystem, addressing core responsibilities, resource allocation, sustainable safe handling, segregation and disposal of medical device waste is likely to a long-term process, sustained by gradual incremental improvements and by increased stakeholder engagements. This integrated approach is likely to be supported and enabled by effective tailored strategies and systems, along with strong oversight and regulation, with the ultimate goal of informing national and international appropriate standards.
- Open Access
- Review
- Neil J. Rowan 1, 2
Author Information
Received: 18 Apr 2025 | Revised: 29 May 2025 | Accepted: 10 Jun 2025 | Published: 16 Jun 2025
Abstract
Graphical Abstract
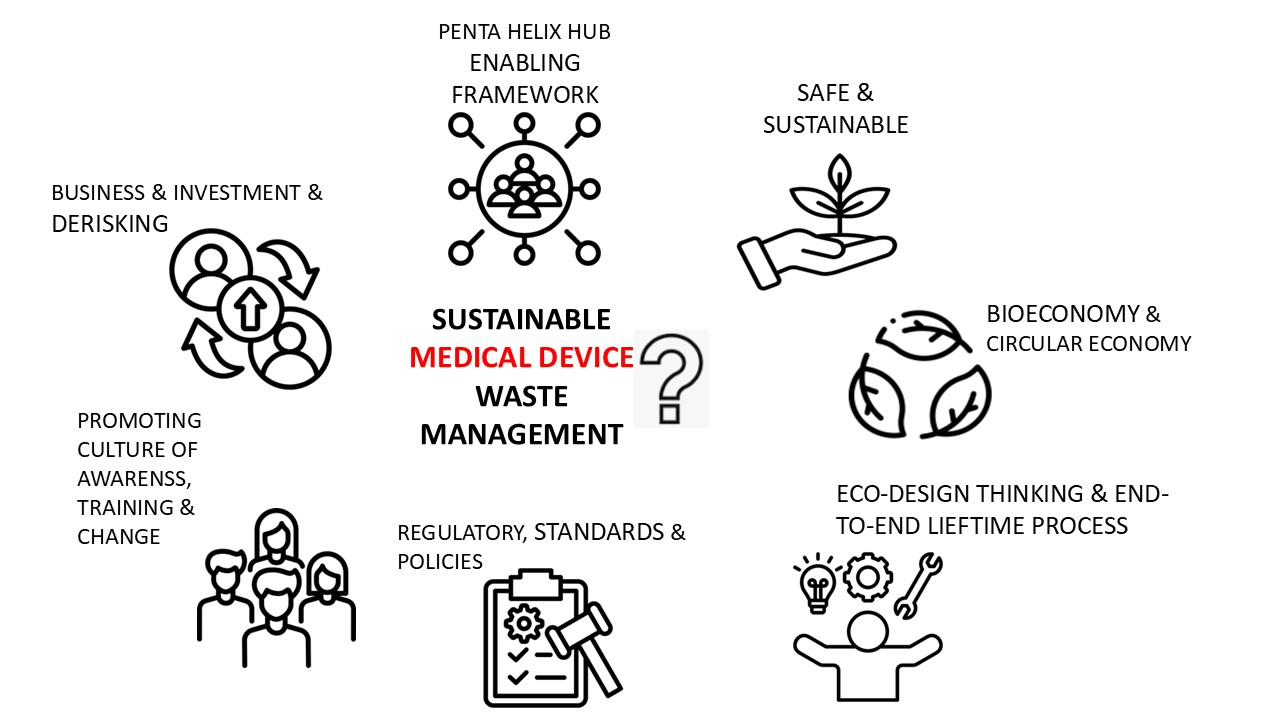
Keywords
medical devices | reuse | sustainability | resource management | circularity | patient safety
References
- 1.
Rowan, N.J.; Laffey, J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic—Case study from the Republic of Ireland. Sci. Total Environ. 2020, 725, 138532. https://doi.org/10.1016/j.scitotenv.2020.138532.
- 2.Rowan, N.J.; Moral, R.A. Disposable face masks and reusable face coverings as non-pharmaceutical interventions (NPIs) to prevent transmission of SARS-CoV-2 variants that cause coronavirus disease (COVID-19): Role of new sustainable NPI design innovations and predictive mathematical modelling. Sci. Total Environ. 2021, 772, 145530. https://doi.org/10.1016/j.scitotenv.2021.145530.
- 3.WHO Tonnes of COVID-19 Health Care Waste Expose Urgent Need to Improve Waste Management Systems. 2022. Available online: https://www.who.int/news/item/01-02-2022-tonnes-of-covid-19-health-care-waste-expose-urgent-need-to-improve-waste-management-systems (accessed on 29 May 2025).
- 4.Rowan, N.J. Challenges and future opportunities to unlock the critical supply chain of personal and protective equipment (PPE) encompassing decontamination and reuse under emergency use authorization (EUA) conditions during the COVID-19 pandemic: Through a reflective circularity and sustainability lens. Sci. Total Environ. 2023, 866, 161455. https://doi.org/10.1016/j.scitotenv.2023.161455.
- 5.Rowan, N.J. Embracing a Penta helix hub framework for co-creating sustaining and potentially disruptive sterilization innovation that embraces artificial intelligence and sustainability: A scoping review. Sci. Total Environ. 2025, 972, 179018. https://doi.org/10.1016/j.scitotenv.2025.179018.
- 6.WHO. Health-Care Waste. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/health-care-waste (accessed on 29 May 2025).
- 7.Zhou, T.; Wu, J.; Hu, X.; et al. Microplastics released from disposable medical devices and their toxic responses in Caenorhabditis elegans. Environ. Res. 2023, 239, 117345. https://doi.org/10.1016/j.envres.2023.117345.
- 8.Rowan, N.J. Peatlands-based demonstration of bioeconomy innovations at scale to help achieve many of the United Nation’s Sustainable Development Goals. Resour. Environ. Sustain. 2025, 21, 100238. https://doi.org/10.1016/j.resenv.2025.100238.
- 9.Hoveling, T.; Nijdam, A.S.; Moninex, M.; et al. Circular economy for medical devices: Barriers, opportunities and best practices from a design perspective. Resour. Conserv. Recycl. 2024, 208, 107719. https://doi.org/10.1016/j.resconrec.2024.107719.
- 10.Moreno, M.; De los Rios, C.; Rowe, Z.; et al. A conceptual framework for circular design. Sustainability 2016, 8, 937. https://doi.org/10.3390/su8090937.
- 11.Kane, G.M.; Bakker, C.A.; Balkenende, A.R. Towards design strategies for circular medical products. Resour. Conserv. Recycl. 2018, 135, 38–47. https://doi.org/10.1016/j.resconrec.2017.07.030.
- 12.Rowan, N.; Kremer, T.; McDonnell, G. A review of Spaulding’s Classification System for Effective Cleaning, Disinfection, Sterilization or Reusable Medical Devices: Viewed through a modern-day lens that will inform and enable future sustainability. Sci. Total Environ. 2023, 878, 162976. https://doi.org/10.1016/j.scitotenv.2023.165673.
- 13.Chua, R.A.H.W.; Lim, S.K.; Chee, C.F.; et al. Surgical site infection and development of antimicrobial sutures: A review. Eur. Rev. Med. Pharm. Sci. 2022, 26, 828–845.
- 14.Patal, P.K.; Advani, S.D.; Kofman, A.D.; et al. Strategies to prevent catheter-associated urinary tract infections in acute-care hospitals: 2022 update. Infect. Control Hosp. Epidemiol. 2023, 44, 1209–1231.
- 15.Dadi, N.C.T.; Radochová, B.; Vargová, J.; et al. Impact of Healthcare-Associated Infections Connected to Medical Devices-An Update. Microorganisms 2021, 9, 2332. https://doi.org/10.3390/microorganisms9112332.
- 16.Whelan, J. Current issues in reprocessing of medical and surgical instruments. Am. J. Infect. Control 2023, 51, 1185–1188.
- 17.Kremer, T.; Rowan, N.; McDonnell, G. A New Quantitative Method for Determining Patient Risk for Reusable Medical Devices Catagorization Based on Using and Interpreting Kremer’s Cleaning Classification System. J. Hosp. Infect. 2024, 155, 234–247. https://doi.org/10.1016/j.jhin.2024.09.024.
- 18.ISO 11139; Sterilization of Heath Care Products—Vocabulary. International Organization for Standardization: Geneva, Switzerland, 2018.
- 19.Smith, A.; Bancroft, R.; Ingle, D.; et al. Misinterpretation of medical device cleaning standards. J. Hosp. Infect. 2023, 135, 199–200. htts://doi.org/10.1016/j.hin.2023.02.009.
- 20.Kremer, T.; Rowan, N.J.; McDonnell, G. A proposed cleaning classification system for reusable medical devices to complement the Spaulding Classification. J. Hosp. Infect. 2023, 145, 88–98. https://doi.org/10.1016/j.jhin.2023.11.018.
- 21.Garvey, M. Medical device-associated healthcare infections: Sterilization the potential of novel biological approaches to ensure patient safety. Int. J. Mol. Sci. 2024, 25, 201. https//doi.org/10.3390/ijms25010201.
- 22.Ines Silva, M.; Malitckii, E.; Santos, T.G; et al. Review of conventional and advanced non-destructive testing techniques for detection and characterization of small scale defects. Prog. Mater. Sci. 2023, 138, 101155. https://doi.org/10.1016/j.pmatsci.2023.101155.
- 23.Ibn-Mohammed, T.; Mustapha, K.B.; Abdulkareem, K.; et al. Toward artificial intelligence and machine learning-enabled frameworks for improved predictions of lifecycle environmental impacts of functional materials and devices. MRS Commun. 2023, 13, 795–811. https://doi.org/10.1557/s43579-023-00480-w.
- 24.Joshi, G.; Jain, A.; Araveeti, S.R.; et al. FDA-Approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape. Electronics 2024, 13, 498. https://doi.org/10.3390/electronics13030498.
- 25.Rowan, N.J. Digital technologies to unlock safe and sustainable opportunities for medical device and healthcare sectors with a focus on the combined use of digital twin and extended reality applications: A review. Sci. Total Environ. 2024, 826, 171672. https://doi.org/10.1016/j.scitotenv.2024.171672.
- 26.Kremer, T.; Murray, N.; Buckley, J.; et al. Use of real-time immersive digital training and educational technologies to improve patient safety during the processing of reusable medical devices: Quo Vadis? Sci. Total Environ. 2023, 900, 165673.
- 27.Greene, J.; Skolnik, C.L.; Merritt, M.W. How medicine becomes trash: Disposability in health care. Lancet 2022, 400, 1298–1299.
- 28.Schluep, M.; Minheere, M.; Baus, M.; et al. Reducing plastic waste in intensive care from longer use of intravenous administration and invasive monitoring sets: A before and after study. J. Crit. Care 2024, 84, 154900. https://doi.org/10.1016/j.jcrc.2024.154900.
- 29.Sedgwick’s 2025 State of National Device Recall Index report. Available online: https://marketing.sedgwick.com/acton/media/4952/US-Recall-Index-2025 (accessed on 11 June 2025).
- 30.US Food and Drug Administration (FDA). Medical Device Reporting (MDR): How to Report Medical Device Problems. 2025. Available online: https://www.fda.gov/medical-devices/medical-device-safety/medical-device-reporting-mdr-how-report-medical-device-problems#:~:text=as%20Biological%20Products.-,Voluntary%20Medical%20Device%20Reporting,and%20Adverse%20Event%20Reporting%20Program (accessed on 1 March 2025).
- 31.Kagoma, Y.; Stall, N.; Rubinstein, E.; et al. People, planet and profits: The case for greening operating rooms. CMAJ 2012, 184, 1905–1911.
- 32.Glauser, W.; Petch, J.; Pendharkar, S. Are Disposable Hospital Supplies Trashing the Environment? Healthdebate 2016. Available online: https://healthdebate.co/2016/08topic/hospital-medical-waste/ (accessed on 27 February 2025).
- 33.Pickler, P.P.; Jaccard, J.S.; Weisz, U.; et al. International comparison of health care carbon footprints. Environ. Res. Lett. 2019, 14, 064004.
- 34.Friedericy, H.; van Egmond, C.W.; Vogtlander, J.G.; et al. Reducing the environmental impact of sterilization packaging for surgical instruments in the operating room: A comparative life cycle assessment of disposable versus reusable systems. Sustainability 2022, 14, 430. https://doi.org/10.10.3390/su14010430.
- 35.Environmental Protection Agency—GreenHealthcare. Reducing Waste in Irish Healthcare Facilities: Results, Guidance, and Tips from a Waste Prevention Programme; Environmental Protection Agency: Washington, DC, USA, 2024.
- 36.Wall, M. Frantic PPE Procurement at Height of COVID Crisis to be Reviewed. The Irish Times. 2023. Available online: https://www.irishtimes.com/news/ireland/irish-news/frantic-ppe-procurement-at-height-of-covid-crisis-to-be-reviewed-1.4664375 (accessed on 27 February 2024).
- 37.Irish Examiner. State Paid ‘Astronomical’ €915m for PPE in 2020. 2021. Available online: https://www.irishexaminer.com/news/arid-40313897.html (accessed on 8 November 2024).
- 38.Hansen, J.; Weiss, S.; Kremer, T.; et al. Dry Heat Processing of Single-Use Respirators and Surgical Masks. Biomed. Instrum. Technol. 2020, 54, 410–416.
- 39.Alt, J.; Eveland, R.; Fiorello, A.; et al. Development and validation of technologies suitable for the decontamination and re-use of contaminated N95 filtering facepiece respirators in response to the COVID-19 pandemic. J. Hosp. Infect. 2022, 119, 141–148.
- 40.McGain, F.; Story, D.; Lim, T.; et al. Financial environmental costs of reusable single-use anaesthetic equipment. Br. J. Anaesth. 2017, 118, 862–869.
- 41.McGain, F.; McAlister, S. Reusable versus single-use ICU equipment: What’s the environmental footprint? Intensive Care Med. 2023, 49, 1523–1525.
- 42.Disruptive Technologies Innovation Fund (DTIF) Ireland. Available online: https://enterprise.gov.ie/en/what-we-do/innovation-research-development/disruptive-technologies-innovation-fund/ (accessed on 11 June 2025).
- 43.
HIHI Greentech in Healthcare. GreenTech in Healthcare Represents a Growing Movement within the Global Health Industry to Adopt Technologies and Practices That Are Environmentally Sustainable, Aiming to Improve Health Outcomes While Minimising Ecological Footprints. 2025. Available online: https://hih.ie/initiatives/greentech/#:~:text=GreenTech%20in%20healthcare%20represents%20a,outcomes%20while%20minimising%20ecological%20footprints (accessed on 17 April 2025).
- 44.Association for the Advancement of Medical Instrumentation. ANSI/AAMI ST98 Cleaning Validation of Health Care Products—Requirements for Medic Devices; AAMI: Arlington, VA, USA, 2022.
- 45.Spaulding, E. Chemical disinfection of medical and surgical materials. In Block Disinfection, Sterilization, and Preservation; Lea & Febiger: Philadelphia, PA, USA, 1968; pp. 517–531.
- 46.Ofstead, C.L.; Hopkins, K.M.; Buro, B.L.; et al. Challenges in achieving effective high-level disinfection in endoscope reprocessing. Am. J. Infect. Control 2020, 48, 309–315.
- 47.Murray, N.; Buckley, J.; Seery, N.; et al. Blending immersive and educational technologies to inform sustainability and diversification of workforce training through machine interface using sterilization as model—Quo vadis? In Proceedings of the 10th Kilmer Conference, Dublin, Ireland, 3–6 June 2019. https://research.thea.ie/handle/20.500.12065/3280.
- 48.Williams, D.; Dignley, J.; Jones, C.; et al. Contamination of laryngoscope handles. J. Hosp. Infect. 2010, 74, 123–128.
- 49.Davis, J. Retained bioburden on surgical instruments after reprocessing: Are we just scraping the surface. Pennyslvania Patient Saf. Advis. 2017, 14, 71–75.
- 50.Okamoto, N.; Sczaniecka, A.; Hirano, M.; et al. A perspective, multicentre, clinical study of duodenoscope contamination after reprocessing. Infect. Control Hosp. Epidemiol. 2022, 43, 1901–1909.
- 51.Kremer, T.; Felgar, J.; Rowan, N.; et al. Validation of the device feature approach for reusable medcial device cleaning evaluations. Biomed. Biomed. Instrum. Technol. 2023, 57, 143–152. https://doi.org/10.2345/0899-8205-57.4.143.
- 52.Martins, R.S.; Salar, H.; Salar, M.; et al. Making minimally invasive procedures more sustainable: A systematic review comparing the environmental footprint of single-use versus multi-use instruments. World J. Surg. 2024, 48, 2212–2223. https://doi.org/10.1002/wjs.12286.
- 53.Donahue, L.M.; Petit, H.J.; Thiel, C.L.; et al. A Life Cycle Assessment of Reusable and Disposable Surgical Caps. J. Surg. Res. 2024, 299, 112–119. https://doi.org/10.1016/j.jss.2024.04.007.
- 54.López-Muñoz, P.; Martín-Cabezuelo, R.; Lorenzo-Zúñiga, V.; et al. Environmental footprint and material composition comparison of single-use and reusable duodenoscopes. Endoscopy 2025, 57, 116–123. https://doi.org/10.1055/a-2364-1654.
- 55.Thone, M.; Lask, J.; Hennenlotter, J.; et al. Potential impacts to human health from climate change: A comparative life-cycle assessment of single-use versus reusable devices flexible ureteroscopes. Urolithiasis 2024, 32, 255. https://doi.org/10.1007/s00240-024-01664-2.
- 56.Duffy, J.; Slutzman, J.E.; Thiel, C.L.; et al. Sustainable Purchasing Practices: A Comparison of Single-use and Reusable Pulse Oximeters in the Emergency Department. West. J. Emerg. Med. 2023, 24, 1034–1042. https://doi.org/10.5811/westjem.58258.
- 57.Lichtnegger, S.; Meissner, M.; Paolini, F.; et al. Comparative Life Cycle Assessment Between Single-Use and Reprocessed IPC Sleeves. Risk Manag. Healthc. Policy 2023, 16, 2715–2726. https://doi.org/10.2147/RMHP.S439982.
- 58.Nabi, Z.; Tang, R.S.Y.; Sundaram, S.; et al. Single-use accessories and endoscopes in the era of sustainability and climate change-A balancing act. J. Gastroenterol. Hepatol. 2024, 39, 7–17. https://doi.org/10.1111/jgh.16380.
- 59.Sherman, J.D.; Raibley, L.A.; Eckelman, M.J. Life Cycle Assessment and Costing Methods for Device Procurement: Comparing Reusable and Single-Use Disposable Laryngoscopes. Anesth. Analg. 2018, 127, 434–443. https://doi.org/10.1213/ANE.0000000000002683.
- 60.Thiel, C.L.; Eckelman, M.; Guido, R.; et al. Environmental impacts of surgical procedures: Life cycle assessment of hysterectomy in the United States. Environ. Sci. Technol. 2015, 49, 1779–1786. https://doi.org/10.1021/es504719g.
- 61.Byrne, D.; Saget, S.; Davidson, A.; et al. Comparing the environmental impact of reusable and disposable dental examination kits: A life cycle assessment approach. Br. Dent. J. 2022, 233, 317–325. https://doi.org/10.1038/s41415-022-4912-4.
- 62.Siu, J.; Hill, A.G.; MacCormick, A.D. Systematic review of reusable versus disposable laparoscopic instruments: Costs and safety. ANZ J. Surg. 2017, 87, 28–33. https://doi.org/10.1111/ans.13856. https://doi.org/10.1017/ice.2025.41.
- 63.Ghorai, R.P.; Kumar, R. Reuse of Single-Use Devices in Endourology: A Review. J. Endourol. 2024, 38, 68–76. https://doi.org/10.1089/end.2023.0367.
- 64.Chu, J.; Ghenand, O.; Collins, J.; et al. Thinking green: Modelling respirator reuse strategies to reduce cost and waste. BMJ Open 2021, 11, e048687. https://doi.org/10.1136/bmjopen-2021-048687.
- 65.Murphy, A.; Howlett, D.; Gowson, A.; et al. Understanding the feasibility and environmental effectiveness of a pilot postal inhaler recovery and recycling scheme. NPJ Prim. Care Respir. Med. 2023, 33, 5. https://doi.org/10.1038/s41533-023-00327-w.
- 66.Tan, H.; Feng, B.; Liu, Y.; et al. Disposal and application of discarded nitrile gloves in sustainable cement-based materials. 2025, 12, 1579229. https://doi.org/10.3389/fmats.2025.1579229.
- 67.US Department of Health and Human Services Food and Drug Administration. Labeling Recommendations for Single-Use Devices Reprocessed by Third Parties and Hospitals; Final Guidance for Industry and FDA; US Department of Health and Human Services: Silver Spring, MD, USA, 2001.
- 68.MHRA New Guidance and Information for Industry for the MHRA. Available online: https://www.gov.uk/government/collections/new-guidance-and-information-for-industry-from-the-mhra (accessed on 11 June 2025).
- 69.Vukelich, D. Reprocessing Remanufacturing of Single-Use Medical Devices. In Block’s Disinfection, Sterilization, and Preservation; Wolters Kluwer: Philadelphia, PA, USA, 2021; pp. 1145–1151.
- 70.McGrane, V.; McDermott, O.; Trubetskaya, A.; et al. The effect of medical device regulations on deploying a Lean Six Sigma project. Processes 2022, 10, 2303. https://doi.org/10.3390pr10112303.
- 71.Chen, Y.; Xu, Z.; Wang, X.; et al. How does green credit policy improve corporate social responsibility in China? An analysis based on carbon-intensive listed firms. Corp. Soc. Responsib. Environ. Manag. 2023, 30, 889–904.
- 72.Denny, N.A.; Guyer, J.M.; Schroeder, D.R.; et al. Operating Room Waste Reduction. AANA J. 2019, 87, 477–482.
- 73.Ordway, A.; Pitonyak, J.S.; Johnson, K.L. Durable medical equipment reuse and recycling: Uncovering hidden opportunities for reducing medical waste. Disabil. Rehabil. Assist. Technol. 2020, 15, 21–28. https://doi.org/10.1080/17483107.2018.1508516.
- 74.McGain, F.; Muret, J.; Lawson, C.; et al. Environmental sustainability in anaesthesia and critical care. Br. J. Anaesth. 2020, 125, 680–692. https://doi.org/10.1016/j.bja.2020.06.055.
- 75.Laustsen, G. Reduce--recycle--reuse: Guidelines for promoting perioperative waste management. AORN J. 2007, 85, 717–728. https://doi.org/10.1016/S0001-2092(07)60146-X.
- 76.Ghersin, Z.J.; Flaherty, M.R.; Yager, P.; et al. Going green: Decreasing medical waste in a paediatric intensive care unit in the United States. New Bioeth. 2020, 26, 98–110. https://doi.org/10.1080/20502877.2020.1767916.
- 77.Boccato, C.; Vienken, J. Do medical devices contribute to sustainability? Environmental, societal and governance aspects. Int. J. Artif. Organs 2024, 47, 229–239. https://doi.org/10.1177/03913988241245015.
- 78.Vienken, J.; Boccato, C. Do medical devices contribute to sustainability? The role of innovative polymers and device design. Int. J. Artif. Organs. 2024, 47, 240–250. https://doi.org/10.1177/03913988241245013.
- 79.Zhou, H.; Yu, X.; Alhaskawi, A.; et al. A deep learning approach for medical waste classification. Sci. Rep. 2022, 12, 2159. https://doi.org/10.1038/s41598-022-06146-2.
- 80.Al-Omran, K.; Khan, E. Predicting medical waste generation and associated factors using machine learning in the Kingdom of Bahrain. Environ. Sci. Pollut. Res. 2024, 31, 38343–38357. https://doi.org/10.1007/s11356-024-33773-1.
- 81.Singh, N.; Ogunseitan, O.A.; Tang, Y. Medical waste: Current changes and future opportunities for sustainable management. Crit. Rev. Environ. Sci Technol. 2021, 52, 2000–2022. https://doi.org/10.1080/10643389.2021.1885325.
- 82.Rizan, C.; Bhutta, M.F. Environmental impact and life cycle financial cost of hybrid (resuable/single-use) instruments versus single-use equivalents in laparoscopic cholecystectomy. Surg. Endosc. 2022, 36, 4067–4078.
- 83.Sudiana, K.; Sule, E.T.; Soemaryani, I. The development and validation of the penta helix construct. Bus. Theory Pract. 2020, 21, 136–145.
- 84.McLaren, J. Medical Device Sterilization modality selection decision process. Biomed. Instrum. Technol. 2020, 54, 6–14. https://doi.org/10.2345/0899-8205-54.s3.6.
- 85.McLaren, J.; Hansen, J.M.; Le, V. Sterilization modality selection: Role of Sterilization Assurance Subject Matter expert. Biomed. Instrum. Technol. 2021, 55, 67–77. https://doi.org/10.2345/0899-8205-55.s3.67.
- 86.Smith, M.; Singh, H.; Sherman, J.D. Infection prevention, planetary health, and single-use plastics. JAMA 2023, 330, 1947–1948.
- 87.Potting, J.; Hekkert, M.; Worrell, E.; et al. 2017. Circular Economy: Measuring Innovation in the Product Chain; The Hague PBL Netherlands Assessment Agency: Den Haag, The Netherlands.
- 88.Rowan, N.J.; Casey, O. Empower Eco multiactor HUB: A triple helix ‘academia-industry-authority’ approach to creating and sharing potentially disruptive tools for addressing novel and emerging new Green Deal opportunities under a United Nations Sustainable Development Goals framework. Curr. Opin. Environ. Sci. Health 2021, 21, 100254. https://doi.org/10.1016/j.coesh.2021.100254.
- 89.Rowan, N.J. Pulsed light as an emerging technology to cause disruption for food and adjacent industries—Quo Vadis? Trend. Food. Sci. Technol. 2019, 88, 316–332.
- 90.Ofstead, C.L.; Smart, A.G.; Hurst, L.L.; et al. Endoscope processing effectiveness: A reality check and call to action for infection preventionists and clinicians. Am. J. Infect. Control 2025, in press. https://doi.org/10.1016/j.ajic.2025.04.003.
- 91.Anukwonke, C.C.; Abazu, C.I. Green Business through Carbon Credits. In Climate Change Alleviation for Sustainable Progression; CRC Press: Boca Raton, FL, USA, 2022; pp. 315–332.
- 92.Tu, X.; Autiosalo, J.; Ala-Laurinaho, R.; et al. TwinXR: Method for using digital twin descriptions in industrial eXtended reality applications. Front. Virtual Real. 2023, 4, 1019080. https://doi.org/10.3389/frvir.2023.1019080.
- 93.Sun, T.; He, X.; Li, Z. Digital twin in healthcare: Recent updates and challenges. Digit Health. 2023, 3, 9. https://doi.org/10.1177/20552076221149651.
- 94.Chen, Y.; Neff, M.; McEvoy, B.; et al. 3D printed polymers are less stable than injection moulded counterparts when exposed to terminal sterilization processes using novel vaporized hydrogen peroxide and electron beam processes. Polymer 2019, 183, 121870. https://doi.org/10.1016/j.polymer.2019.121870.
- 95.Valls-Esteve, A.; Lustig-Gainza, P.; Adell-Gomez, N.; et al. A state-of-the-art guide about the effects of sterilization processes on 3D-printed materials for surgical planning and medical applications: A comparative study. Int. J. Bioprinting 2023, 9, 756. https://doi.org/10.18063/ijb.756.
- 96.Kanschik, D.; Bruno, R.R.; Wolff, G.; et al. Virtual and augmented reality in intensive care medicine: A systematic review. Ann. Intensive Care 2023, 13, 81. https://doi.org/10.1186/s13613-023-01176-z.
- 97.Boedecker, C.; Huettl, F.; Saalfeld, P.; et al. Using virtual 3D-models in surgical planning: Workflow of an immersive virtual reality application in liver surgery. Langenbecks Arch. Surg. 2021, 406, 911–915. https://doi.org/10.1007/s00423-021-02127-7.
- 98.Viceconti, M.; De Vos, M.; Mellone, S.; et al. Position paper from the digital twins in healthcare to the Virtual Human Twin: A moon-shot project for digital health research. IEEE J. Biomed. Health Inform. 2023, 28, 491–501. https://doi.org/10.1109/JBHI.2023.3323688.
- 99.Kamel Boulos, M.N.; Zhang, P. Digital Twins: From Personalised Medicine to Precision Public Health. J. Pers. Med. 2021, 11, 745. https://doi.org/10.3390/jpm11080745.

This work is licensed under a Creative Commons Attribution 4.0 International License.


