Dynamic shifts in global temperatures have increased the intensity of extreme weather events over many decades, leading to an increase in wildfires, drought, floods, intense hurricanes, longer hurricane seasons, damaging dust storms, humidity changes, decreasing foliage canopy, and altering crop patterns. Accelerated environmental changes can cause negative impacts on everyday human activities and living conditions leading to an increase in the likelihood of human exposure to anthropogenic chemicals: i.e., microplastics; insecticides, fungicides, and herbicides; and biological chemicals: i.e., algal toxins, these exposures are defined collectively as the “exposome”. Every human being is unique in their genetic makeup; therefore, individuals will respond differently to those chemical exposures. Intersecting with climate change is a global increase in neurodegenerative disorders. Exposure to specific compounds has been linked to various neurological diseases, such as dementia, Alzheimer’s, and Parkinson’s.
- Open Access
- Review
Climate Change, the Exposome and the Rising Burden of Neurodegenerative Diseases: A Review
- Hamed Heidari 1,
- Tammy Jones-Lepp 2, *,
- MayaRae N. Mugosa 2,
- Kathryn Woods 2,
- Eakalak Khan 1,
- Erica J. Marti 1,
- Jefferson W. Kinney 2
Author Information
Received: 19 Jun 2025 | Revised: 30 Aug 2025 | Accepted: 01 Sep 2025 | Published: 04 Sep 2025
Abstract
Graphical Abstract
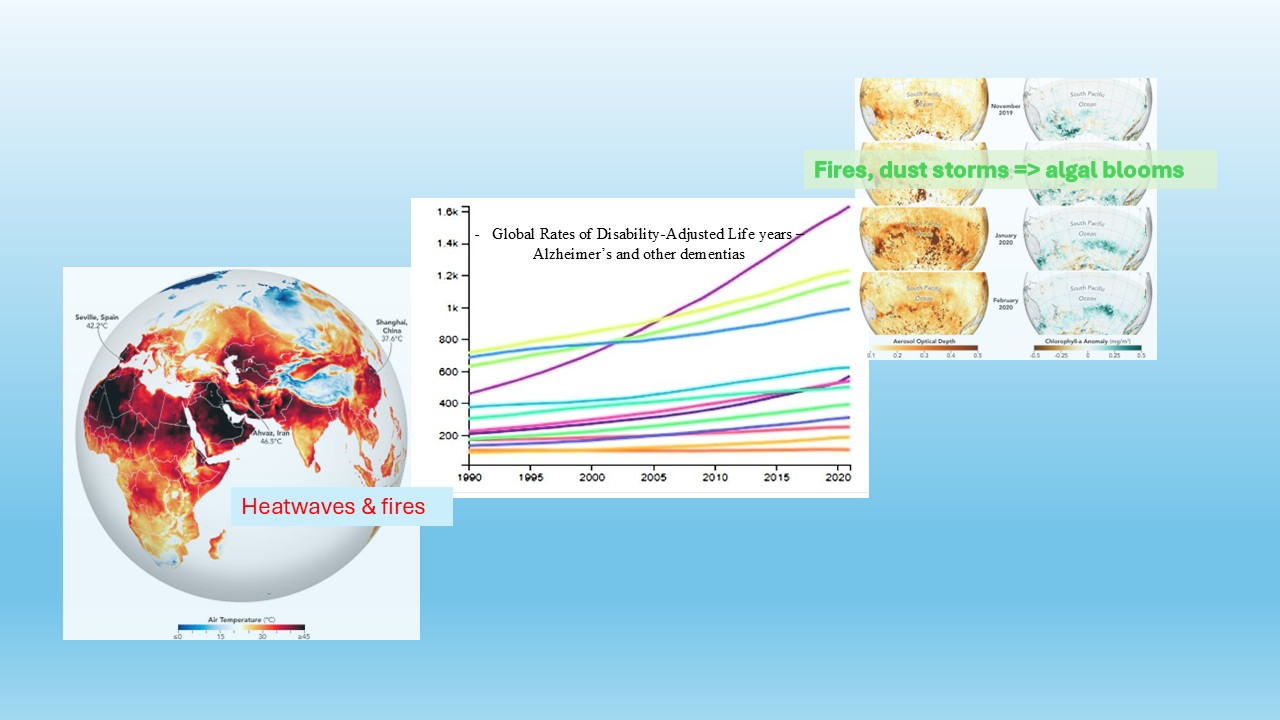
Keywords
microplastics | pesticides | algal toxins | Alzheimer’s | Parkinson’s | ALS | climate change
References
- 1.NOAA National Centers for Environmental Information (2025). Global Climate Report June 2025. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/202506/2025-year-to-date-temperatures-versus-previous-years (accessed on 8 August 2025).
- 2.Abbass, K.; Qasim, M.Z.; Song, H.; et al. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. https://doi.org/10.1007/s11356-022-19718-6.
- 3.Chen, W.; Liu, L.; Liu, D.; et al. Droughts and windstorms due to climate change increase variability in species and trait composition of a subtropical monsoon evergreen broadleaf forest in China. For. Ecosyst. 2025, 12, 100253. https://doi.org/10.1016/j.fecs.2024.100253.
- 4.Centre for Research on the Epidemiology of Disasters (CRED). 2024 Disasters in Numbers; CRED: Brussels, Belgium, 2024. Available online: https://files.emdat.be/reports/2024_EMDAT_report.pdf (accessed on 14 August 2025).
- 5.Rocque, R.J.; Beaudoin, C.; Ndjaboue, R.; et al. Health effects of climate change: An overview of systematic reviews. BMJ Open 2021, 11, e046333. https://doi.org/10.1136/bmjopen-2020-046333.
- 6.Franchini, M.; Mannucci, P.M. Impact on human health of climate changes. Eur. J. Intern. Med. 2014, 26, 1–5. https://doi.org/10.1016/j.ejim.2014.12.008.
- 7.Watts, N.; Amann, M.; Arnell, N.; et al. The 2020 report of the Lancet Countdown on health and climate change: Responding to converging crises. Lancet 2021, 397, 129–170. https://doi.org/10.1016/S0140-6736(20)32290-X.
- 8.World Meteorological Organization (WMO). Global Annual to Decadal Climate Update 2025–2029; WMO: Geneva, Switzerland, 2025. Available online: https://wmo.int/sites/default/files/2025-05/WMO_GADCU_2025-2029_Final.pdf (accessed on 14 August 2025).
- 9.Available online: https://coast.noaa.gov/states/fast-facts/hurricane-costs.html (accessed on 14 August 2025).
- 10.Available online: https://www.ncei.noaa.gov/access/billions/events/US/1980-2024/?disasters[]=tropical-cyclone (accessed on 14 August 2025).
- 11.Young, R., Hsiang, S. Mortality caused by tropical cyclones in the United States. Nature 2024, 635, 121–128. https://doi.org/10.1038/s41586-024-07945-5.
- 12.Available online: https://archive.cdc.gov/www_cdc_gov/niosh/topics/exposome/default (accessed on 14 August 2025).
- 13.Vineis, P.; Robinson, O.; Chadeau-Hyam, M.; et al. What is new in the exposome? Environ. Int. 2020, 143, 105887. https://doi.org/10.1016/j.envint.2020.105887.
- 14.Nabi, M.; Tabassum, N. Role of environmental toxicants on neurodegenerative disorders. Front. Toxicol. 2022, 4, 837579. https://doi.org/10.3389/ftox.2022.837579.
- 15.Lefèvre-Arbogast, S.; Chaker, J.; Mercier, F.; et al. Assessing the contribution of the chemical exposome to neurodegenerative disease. Nat. Neurosci. 2024, 27, 812–821. https://doi.org/10.1038/s41593-024-01627-1.
- 16.Huang, Y.; Li, Y.; Pan, H.; et al. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J. Glob. Health. 2023, 13, 04160. https://doi.org/10.7189/jogh.13.04160.
- 17.Available online: https://www.alz.org/getmedia/ef8f48f9-ad36-48ea-87f9-b74034635c1e/alzheimers-facts-and-figures.pdf (accessed on 14 August 2025).
- 18.Nguyen, T.A.; Pham, T.; Dang, T.H.; et al. Towards the development of Vietnam’s national dementia plan-the first step of action. Australas J. Ageing 2020, 39, 137–141. https://doi.org/10.1111/ajag.12755.
- 19.Lee, J.; Meijer, E.; Langa, K.M.; et al. Prevalence of dementia in India: National and state estimates from a nationwide study. Alzheimer’s Dement. 2023, 19, 2898–2912. https://doi.org/10.1002/alz.12928.
- 20.Gobler, C.J. Climate change and harmful algal blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. https://doi.org/10.1016/j.hal.2019.101731.
- 21.Moore, S.K.; Trainer, V.L.; Mantua, N.J.; et al. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, 1–12. https://doi.org/10.1186/1476-069X-7-S2-S4.
- 22.Rogers, M.M.; Stanley, R.K. Airborne Algae: A Rising Public Health Risk. Environ. Sci. Technol. 2023, 57, 5501–5503. https://doi.org/10.1021/acs.est.3c01158.
- 23.Sini, P.; Dang, T.B.C.; Fais, M.; et al. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons. Int. J. Mol. Sci. 2021, 22, 8726. https://doi.org/10.3390/ijms22168726.
- 24.Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; et al. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. https://doi.org/10.1038/s41579-023-00900-7.
- 25.Chen, X.; Shi, G.; Zhong, G.; et al. Triphenyltin inhibition of the proteasome activity and its influence on substrate protein levels in nerve cells. Chin. Sci. Bull. 2010, 55, 22–26. https://doi.org/10.1007/s11434-009-0678-1.
- 26.Hu, D.; Shen, M.; Zhang, Y.; et al. Microplastics and nanoplastics: Would they affect global biodiversity change? Environ. Sci. Pollut. Res. 2019, 26, 19997–20002. https://doi.org/10.1007/s11356-019-05414-5.
- 27.Shi, W.; Wu, N.; Zhang, Z.; et al. A global review on the abundance and threats of microplastics in soils to terrestrial ecosystem and human health. Sci. Tot. Environ. 2024, 912, 169469. https://doi.org/10.1016/j.scitotenv.2023.169469.
- 28.Gou, X.; Fu, Y.; Li, J.; et al. Impact of nanoplastics on Alzheimer’s disease: Enhanced amyloid-β peptide aggregation and augmented neurotoxicity. J. Hazard. Mater. 2024, 465, 133518. https://doi.org/10.1016/j.jhazmat.2024.133518.
- 29.Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. https://doi.org/10.1016/j.toxicon.2009.07.036.
- 30.Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015, 6, 1254. https://doi.org/10.3389/fmicb.2015.01254.
- 31.Rodgers, K.J.; Main, B.J.; Samardzic, K. Cyanobacterial neurotoxins: Their occurrence and mechanisms of toxicity. Neurotox. Res. 2018, 33, 168–177. https://doi.org/10.1007/s12640-017-9757-2.
- 32.Jonasson, S.; Eriksson, L.E.; Berntzon, L.; et al. A novel cyanobacterial toxin (BMAA) with potential neurodegenerative effects. Plant Biotechnol. 2008, 25, 227–232. https://doi.org/10.5511/plantbiotechnology.25.227.
- 33.Torbick, N.; Hession, S.; Stommel, E.; et al. Mapping amyotrophic lateral sclerosis lake risk factors across northern New England. Int. J. Health Geogr. 2014, 13, 1. https://doi.org/10.1186/1476-072X-13-1.
- 34.Hu, Y.; Chen, J.; Fan, H.; et al. A review of neurotoxicity of microcystins. Environ. Sci. Pollut. Res. 2016, 23, 7211–7219. https://doi.org/10.1007/s11356-016-6073-y.
- 35.Mokoena, M.M. Microcystins in water containers used in the home: A review of their potential health effects. Ecotoxicol. Environ. Saf. 2024, 269, 115787. https://doi.org/10.1016/j.ecoenv.2023.115787.
- 36.US EPA, Drinking Water Health Advisory for Microcystins-June 2015, Document EPA-820R15100. Available online: https://www.epa.gov/sites/default/files/2017-06/documents/microcystins-report-2015.pdf (accessed on 14 August 2025).
- 37.Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. (WHO/HEP/ECH/WSH/2020.6). Available online: https://iris.who.int/bitstream/handle/10665/338066/WHO-HEP-ECH-WSH-2020.6-eng.pdf (accessed on 14 August 2025).
- 38.Yan, M.; Jin, H.; Pan, C.; et al. Movement disorder and neurotoxicity induced by chronic exposure to microcystin-LR in mice. Mol. Neurobiol. 2022, 59, 5516–5531. https://doi.org/10.1007/s12035-022-02919-y.
- 39.Wang, J.; Chen, Y.; Zhang, C.; et al. Learning and memory deficits and alzheimer’s disease-like changes in mice after chronic exposure to microcystin-LR. J. Haz. Mater. 2019, 373, 504–518. https://doi.org/10.1016/j.jhazmat.2019.03.106.
- 40.Wang, J.; Zhang, C.; Zhu, J.; et al. Blood-brain barrier disruption and inflammation reaction in mice after chronic exposure to Microcystin-LR. Sci. Total Environ. 2019, 689, 662–678. https://doi.org/10.1016/j.scitotenv.2019.06.387.
- 41.Martin, R.M.; Bereman, M.S.; Marsden, K.C. BMAA and MCLR interact to modulate behavior and exacerbate molecular changes related to neurodegeneration in Larval Zebrafish. Toxicol. Sci. 2020, 179, 251. https://doi.org/10.1093/toxsci/kfaa178.
- 42.Zhao, S.; Xu, J.; Zhang, W.; et al. Paternal exposure to microcystin-LR triggers developmental neurotoxicity in zebrafish offspring via an epigenetic mechanism involving MAPK pathway. Sci. Total Environ. 2021, 792, 148437. https://doi.org/10.1016/j.scitotenv.2021.148437.
- 43.Zhao, S.; Yuan, C.; Tuo, X.; et al. MCLR induces dysregulation of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in Sertoli cells. Chemosphere 2021, 263, 127868. https://doi.org/10.1016/j.chemosphere.2020.127868.
- 44.Yan, M.; Wu, H.; Wu, T.; et al. Microcystin-LR Exposure Damages Neurons by Inducing α-Syn Aggregation via MAPK4/GATA2/SNCA and PP2A/GRKs Pathways. Mol. Neurobiol. 2025, 62, 6195–6211. https://doi.org/10.1007/s12035-024-04683-7.
- 45.Lee, S.E. Guam dementia syndrome revisited in 2011. Curr. Opin. Neurol. 2011, 24, 517–524. https://doi.org/10.1097/WCO.0b013e32834cd50a.
- 46.Colas, S.; Marie, B.; Lance, E.; et al. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ. Res. 2021, 193, 110590. https://doi.org/10.1016/j.envres.2020.110590.
- 47.Kumar, N.; Garg, A. Structural optimization and docking studies of anatoxin-a: A potent neurotoxin. African J. Biotechnol. 2014, 13, 3092–3100. https://doi.org/10.5897/ajb2014.13671.
- 48.Bezprozvanny, I.B. Calcium signaling and neurodegeneration. Acta Naturae 2010, 2, 72–80. https://doi.org/10.32607/20758251-2010-2-1-72-80.
- 49.Campos, F.; Alfonso, M.; Vidal, L.; et al. Mediation of glutamatergic receptors and nitric oxide on striatal dopamine release evoked by anatoxin-a. An in vivo microdialysis study. Eur. J. Pharmacol. 2006, 548, 90–98. https://doi.org/10.1016/j.ejphar.2006.07.044.
- 50.Fernandes, K.A.; Fadul, J.C.; Fiore, M.F.; et al. A systematic review on guanitoxin: General characteristics and ecological risks. Chemosphere 2024, 352, 141277. https://doi.org/10.1016/j.chemosphere.2024.141277.
- 51.Patocka, J.; Gupta, R.C.; Kamil, K. Anatoxin-A(S): Natural organophosphorus anticholinesterase agent. Mil. Med. Sci. Lett. 2011, 80, 129–139. https://doi.org/10.31482/mmsl.2011.019.
- 52.Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. https://doi.org/10.3390/ijms22179290.
- 53.Belykh, O.I.; Tikhonova, I.V.; Kuzmin, A.V.; et al. First detection of benthic cyanobacteria in Lake Baikal producing paralytic shellfish toxins. Toxicon. 2016, 121, 36–40. https://doi.org/10.1016/j.toxicon.2016.08.015.
- 54.Moustaka-Gouni, M.; Hiskia, A.; Genitsaris, S.; et al. First report of Aphanizomenon favaloroi occurrence in Europe associated with saxitoxins and a massive fish kill in Lake Vistonis, Greece. Mar. Freshw. Res. 2017, 68, 793–800. https://doi.org/10.1071/MF16029.
- 55.Suleiman, M.; Jelip, J.; Rundi, C.; et al. Case Report: Paralytic Shellfish Poisoning in Sabah, Malaysia. Am. J. Trop. Med. Hyg. 2017, 97, 1731–1736. https://doi.org/10.4269/ajtmh.17-0589.
- 56.Botelho, M.J.; Milinovic, J.; Bandarra, N.M.; et al. Alzheimer’s Disease and Toxins Produced by Marine Dinoflagellates: An Issue to Explore. Mar. Drugs 2022, 20, 253. https://doi.org/10.3390/md20040253.
- 57.Rhodes, L.A.; McCarl, B.A. An analysis of climate impacts on herbicide, insecticide, and fungicide expenditures. Agron. 2020, 10, 745. https://doi.org/10.3390/AGRONOMY10050745.
- 58.Noyes, P.D.; McElwee, M.K.; Miller, H.D.; et al. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. https://doi.org/10.1016/J.ENVINT.2009.02.006.
- 59.Damalas, C.A. Understanding benefits and risks of pesticide use. Sci. Res. Essays 2009, 4, 945–949.
- 60.Pogacean, M.O.; Gavrilescu, M. Plant protection products and their sustainable and environmentally friendly use. Environ. Eng. Manag. J. 2009, 8, 607–627. https://doi.org/10.30638/eemj.2009.084.
- 61.Żak, A. Plant protection products versus changes in the natural environment and their impact on the human health. Probl. Agric. Econ. 2016, 346, 155–166. https://doi.org/10.30858/zer/83045.
- 62.Kamel, F.; Hoppin, J.A. Association of pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect. 2004, 112, 950–958. https://doi.org/10.1289/ehp.7135.
- 63.Li, G.; Kim, C.; Kim, J.; et al. Common Pesticide, Dichlorodiphenyltrichloroethane (DDT), Increases Amyloid-β Levels by Impairing the Function of ABCA1 and IDE: Implication for Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 46, 109–122. https://doi.org/10.3233/JAD-150024.
- 64.Richardson, J.R.; Roy, A.; Shalat, S.L.; et al. Elevated Serum Pesticide Levels and Risk for Alzheimer Disease. JAMA Neurol. 2014, 71, 284–290. https://doi.org/10.1001/jamaneurol.2013.6030.
- 65.Parrales-Macias, V.; Michel, P.P.; Tourville, A.; et al. The Pesticide Chlordecone Promotes Parkinsonism-like Neurodegeneration with Tau Lesions in Midbrain Cultures and C. elegans Worms. Cells 2023, 12, 1336. https://doi.org/10.3390/cells12091336.
- 66.Alehashem, M.; Alcaraz, A.J.; Hogan, N.; et al. Linking pesticide exposure to neurodegenerative diseases: An in vitro investigation with human neuroblastoma cells. Sci. Tot. Environ. 2024, 933, 173041. https://doi.org/10.1016/j.scitotenv.2024.173041.
- 67.Ferraz da Silva, I.; Freitas-Lima, L.C.; Graceli, J.B.; et al. Organotins in neuronal damage, brain function, and behavior: A short review. Front. Endocrinol. 2018, 8, 366. https://doi.org/10.3389/fendo.2017.00366.
- 68.Baltazar, M.T.; Dinis-Oliveira, R.J.; de Lourdes Bastos, M.; et al. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases—A mechanistic approach. Toxicol. Lett. 2014, 230, 85–103. https://doi.org/10.1016/j.toxlet.2014.01.039.
- 69.Mostafalou, S.; Abdollahi, M. The link of organophosphorus pesticides with neurodegenerative and neurodevelopmental diseases based on evidence and mechanisms. Toxicology 2018, 409, 44–52. https://doi.org/10.1016/J.TOX.2018.07.014.
- 70.Sánchez-Santed, F.; Colomina, M.T.; Hernández, E.H. Organophosphate pesticide exposure and neurodegeneration. Cortex 2016, 74, 417–426. https://doi.org/10.1016/j.cortex.2015.10.003.
- 71.Zaganas, I.; Kapetanaki, S.; Mastorodemos, V.; et al. Linking pesticide exposure and dementia: What is the evidence? Toxicology 2013, 307, 3–11. https://doi.org/10.1016/J.TOX.2013.02.002.
- 72.Tanner, C.M.; Kame, F.; Ross, G.W.; et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011, 119, 866–872. https://doi.org/10.1289/ehp.1002839.
- 73.Lini, R.S.; Scanferla, D.T.P.; de Oliveira, N.G.; et al. Fungicides as a risk factor for the development of neurological diseases and disorders in humans: A systematic review. Crit. Rev. Toxicol. 2024, 54, 35–54. https://doi.org/10.1080/10408444.2024.2303481.
- 74.Barrett, J.R. More concerns for farmers. Neurologic effects of chronic pesticide exposure. Environ. Health Perspect. 2005, 113, A472. https://doi.org/10.1289/ehp.113-a472a.
- 75.Perry, J.; Cotton, J.; Rahman, M.A.; et al. Organophosphate exposure and the chronic effects on farmers: A narrative review. Rural Remote Health 2020, 20, 4508. https://doi.org/10.22605/RRH4508.
- 76.Doi, M.; Usui, N.; Shimada, S. Prenatal environment and neurodevelopmental Disorders. Front. Endocrinol. 2022, 13, 860110. https://doi.org/10.3389/fendo.2022.860110.
- 77.Roberts, J.R.; Dawley, E.H.; Reigart, J.R. Children’s low-level pesticide exposure and associations with autism and ADHD: A review. Pediatr. Res. 2018, 852, 234–241. https://doi.org/10.1038/s41390-018-0200-z.
- 78.Bogaert, E.; d’Ydewalle, C.; Van Den Bosch, L. Amyotrophic lateral sclerosis and excitotoxicity: From pathological mechanism to therapeutic target. CNS. Neurol. Disord. Drug Targets 2010, 9, 297–304. https://doi.org/10.2174/187152710791292576.
- 79.Van Cutsem, P.; Dewil, M.; Robberecht, W.; et al. Excitotoxicity and amyotrophic lateral sclerosis. Neurodegener. Dis. 2005, 2, 147–159. https://doi.org/10.1159/000089620.
- 80.Kamel, F.; Umbach, D.M.; Bedlack, R.S.; et al. Pesticide exposure and amyotrophic lateral sclerosis. Neurotoxicol. 2012, 33, 457–462. https://doi.org/10.1016/j.neuro.2012.04.001.
- 81.Cheng, J.L.; Cook, A.L.; Talbot, J.; et al. How is excitotoxicity being modelled in iPSC-derived neurons? Neurotox. Res. 2024, 425, 1–20. https://doi.org/10.1007/S12640-024-00721-3.
- 82.Tang, K.H. Climate Change and Plastic Pollution: A Review of Their Connections. Trop. Environ. Biol. Technol. 2023, 1, 110–120. https://doi.org/10.53623/tebt.v1i2.341.
- 83.Prüst, M.; Meijer, J.; Westerink, R.H. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. https://doi.org/10.1186/s12989-020-00358-y.
- 84.Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. https://doi.org/10.1016/j.envint.2017.02.013.
- 85.Shen, M.; Huang, W.; Chen, M.; et al. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean Prod. 2020, 254, 120138 https://doi.org/10.1016/j.jclepro.2020.120138.
- 86.Ford, H.V.; Jones, N.H.; Davies, A.J.; et al. The fundamental links between climate change and marine plastic pollution. Sci. Total Environ. 2022, 86, 150392. https://doi.org/10.1016/j.scitotenv.2021.150392.
- 87.Cheung, C.K.H.; Not, C. Impacts of extreme weather events on microplastic distribution in coastal environments. Sci. Total Environ. 2023, 904, 166723. https://doi.org/10.1016/j.scitotenv.2023.166723.
- 88.Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Impact of 2018 Kerala flood on the abundance and distribution of microplastics in marine environment off Cochin, Southeastern Arabian Sea, India. Reg. Stud. Mar. Sci. 2022, 53, 102367. https://doi.org/10.1016/j.rsma.2022.102367.
- 89.Hitchcock, J.N. Storm events as key moments of microplastic contamination in aquatic ecosystems. Sci. Total Environ. 2020, 734, 139436. https://doi.org/10.1016/j.scitotenv.2020.139436.
- 90.Nakajima, R.; Miyama, T.; Kitahashi, T.; et al. Plastic After an Extreme Storm: The Typhoon-Induced Response of Micro- and Mesoplastics in Coastal Waters. Front. Mar. Sci. 2022, 8, 1–11. https://doi.org/10.3389/fmars.2021.806952.
- 91.Paing, Y.M.M.; Eom, Y.; Song, G.B.; et al. Neurotoxic effects of polystyrene nanoplastics on memory and microglial activation: Insights from in vivo and in vitro studies. Sci. Tot. Environ. 2024, 924, 171681. https://doi.org/10.1016/j.scitotenv.2024.171681.
- 92.Ma, M.; Coulon, F.; Tang, Z.; et al. Unveiling the Truth of Interactions between Microplastics and Per- and Polyfluoroalkyl Substances (PFASs) in Wastewater Treatment Plants: Microplastics as a Carrier of PFASs and Beyond. Environ. Sci. Technol. 2025, 59, 2211–2221. https://doi.org/10.1021/acs.est.4c08898.
- 93.Lin, P.; Liu, L.; Ma, Y.; et al. Neurobehavioral toxicity induced by combined exposure of micro/nanoplastics and triphenyltin in marine medaka (Oryzias melastigma). Environ. Pollut. 2024, 356, 124334. https://doi.org/10.1016/j.envpol.2024.124334.
- 94.Cheng, H. Future Earth and Sustainable Developments. Innovation 2020, 1, 100055. https://doi.org/10.1016/j.xinn.2020.100055.
How to Cite
Heidari, H.; Jones-Lepp , T.; Mugosa, M. N.; Woods, K.; Khan, E.; Marti, E. J.; Kinney, J. W. Climate Change, the Exposome and the Rising Burden of Neurodegenerative Diseases: A Review. Earth: Environmental Sustainability 2025, 1 (1), 71–83. https://doi.org/10.53941/eesus.2025.100006.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


