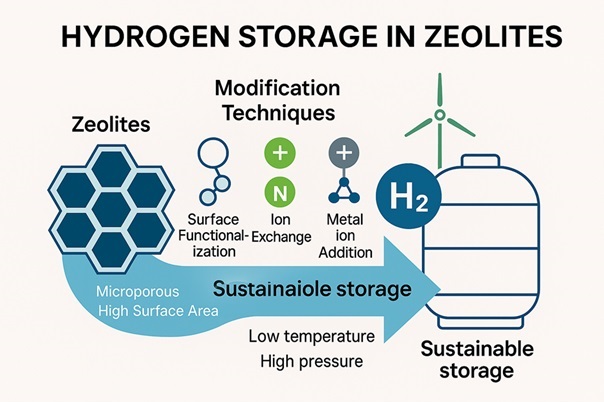
Hydrogen is increasingly being recognized as a clean energy carrier that is vital for decarbonizing industries and integrating renewable energy sources. Efficient hydrogen storage is critical for its widespread adoption and economic viability. Among promising solutions, zeolites have gained attention because of their unique microporous structures, high surface areas, and modifiable chemical properties. These characteristics enable zeolites to effectively adsorb hydrogen molecules, making them suitable for sustainable energy storage and transportation. The exceptional physicochemical properties of zeolites, such as ion exchange and adsorption capacities, allow tailored modifications to enhance their hydrogen storage performance. Techniques such as surface functionalization with amines and ion exchange with specific cations significantly improve adsorption capacity and efficiency. For instance, amine modifications introduce electrostatic interactions, whereas ion exchange optimizes the pore structure and increases the surface charge. Recent studies have highlighted the potential of silver ion-exchanged zeolites for selective hydrogen isotope separation, demonstrating the versatility of these materials. With advancements in zeolite research, the development of scalable, cost-effective, and high-capacity hydrogen storage systems has become increasingly feasible. These innovations position zeolites as key contributors to clean energy transition, supporting the role of hydrogen as a cornerstone of sustainable energy infrastructure.
- Open Access
- Review
Hydrogen Storage in Zeolites: A Mini Review of Structural and Chemical Influences on Adsorption Performance
- Baran Taşğın 1, *,
- Jiří Ryšavý 1,
- Thangavel Sangeetha 2, 3,
- Wei-Mon Yan 2, 3
Author Information
Received: 09 Jan 2025 | Revised: 20 Feb 2025 | Accepted: 22 Feb 2025 | Published: 05 Mar 2025
Abstract
Graphical Abstract
Keywords
hydrogen storage | clean energy carrier | energy sustainability | hydrogen energy sector | decarbonization | renewable energy integration
References
- 1.Vaclav, S. Energy and Civilization: A History; MIT Press: Cambridge, MA, USA, 2017. Available online: https://doi.org/10.7551/mitpress/9780262035774.001.0001 (accessed on 12 December 2024).
- 2.tho Pesch, G.; Einarsdóttir, A.K.; Dillman, K.J.; et al. Energy consumption and human well-being: A Systematic Review. Energies 2023, 16, 6494. https://doi.org/10.3390/en16186494.
- 3.Lee, K.-T.; Cai, Y.-S.; Hou, Q.-Y.; et al. A brief overview of green hydrogen on production, regulations, and commercialization. Green Energy Fuel Res. 2024, 1, 3–12. https://doi.org/10.53941/gefr.2024.100002.
- 4.Ahmad, S.; Ullah, A.; Samreen, A.; et al. Hydrogen production, storage, transportation and utilization for energy sector: A current status review. J. Energy Storage 2024, 101, 113733. https://doi.org/10.1016/j.est.2024.113733.
- 5.IEA. Hydrogen. Available online: https://www.iea.org/energy-system/low-emission-fuels/hydrogen#programmes (accessed on 15 December 2024).
- 6.Li, H.; Cao, X.; Liu, Y.; et al. Safety of hydrogen storage and transportation: An overview on mechanisms, techniques, and challenges. Energy Rep. 2022, 8, 6258–6269. https://doi.org/10.1016/j.egyr.2022.04.067.
- 7.Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. https://doi.org/10.1016/j.est.2021.102676.
- 8.Abdechafik, E.H.; Ousaleh, H.A.; Mehmood, S.; et al. An analytical review of recent advancements on solid-state hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 1182–1193. https://doi.org/10.1016/j.ijhydene.2023.10.218.
- 9.Peng, B.; Chen, J. Functional materials with high-efficiency energy storage and conversion for batteries and fuel cells. Coord. Chem. Rev. 2009, 253, 2805–2813. https://doi.org/10.1016/j.ccr.2009.04.008.
- 10.Database of Zeolite Structures. Available online: https://www.iza-structure.org/databases/ (accessed on 15 December 2024).
- 11.Derbe, T.; Temesgen, S.; Bitew, M. A short review on synthesis, characterization, and applications of zeolites. Adv. Mater. Sci. Eng. 2021, 2021, 6637898. https://doi.org/10.1155/2021/6637898.
- 12.Khaleque, A.; Alam, M.M.; Hoque, M.; et al. A. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. https://doi.org/10.1016/j.envadv.2020.100019.
- 13.Langmi, H.; Walton, A.; Al-Mamouri, M.; et al. Hydrogen adsorption in zeolites A, X, Y and RHO. J. Alloys Compd. 2003, 356–357, 710–715. https://doi.org/10.1016/s0925-8388(03)00368-2.
- 14.Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; et al. Porous materials for hydrogen storage. Chem 2022, 8, 693–716. https://doi.org/10.1016/j.chempr.2022.01.012.
- 15.IndustryARC. Zeolites Market—Forecast (2024–2030). Available online: https://www.industryarc.com/Report/16416/zeolites-market. (accessed on 20 December 2024).
- 16.Dong, J.; Wang, X.; Xu, H.; et al. Hydrogen storage in several microporous zeolites. Int. J. Hydrogen Energy 2007, 32, 4998–5004. https://doi.org/10.1016/j.ijhydene.2007.08.009.
- 17.Anderson, P. Storage of Hydrogen in Zeolites; Elsevier: Amsterdam, The Netherlands, 2008; pp. 223–260. https://doi.org/10.1533/9781845694944.3.223.
- 18.Fraenkel, D. Zeolitic encapsulation. Part 2—Percolation and trapping of inert gases in A-type zeolites. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 2041. https://doi.org/10.1039/f19817702041.
- 19.Salmankhani, A.; Khadem, S.S.M.; Seidi, F.; et al. Adsorption onto zeolites: Molecular perspective. Chem. Pap. 2021, 75, 6217–6239. https://doi.org/10.1007/s11696-021-01817-2.
- 20.Kianfar, E.; Razavi, A. Zeolite catalyst based selective for the process MTG: A review In Zeolites: Advances in Research and Applications. Nova Science Publishers: New York, NY, USA; 2020, pp. 1–39.
- 21.Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in adsorption processes: State of the art and future Prospects. Chem. Rev. 2022, 122, 17647–17695. https://doi.org/10.1021/acs.chemrev.2c00140.
- 22.Sun, Z.; Shu, Q.; Zhang, Q.; et al. A hydrothermal synthesis process of ZSM-5 zeolite for VOCs adsorption using desilication solution. Separations 2024, 11, 39. https://doi.org/10.3390/separations11020039.
- 23.Yue, X.; Wang, S.; Wang, S.; et al. Enhancements on volatile organic compounds (VOCs) adsorption and desorption performance of ZSM-5 by fabricating hierarchical MCM-41. Environ. Sci. Pollut. Res. 2023, 30, 100907–100919. https://doi.org/10.1007/s11356-023-29483-9.
- 24.Mekki, A.; Boukoussa, B. Structural, textural and toluene adsorption properties of microporous–mesoporous zeolite omega synthesized by different methods. J. Mater. Sci. 2019, 54, 8096–8107. https://doi.org/10.1007/s10853-019-03450-7.
- 25.Bahmanzadegan, F.; Pordsari, M.A.; Ghaemi, A. Improving the efficiency of 4A-zeolite synthesized from kaolin by amine functionalization for CO2 capture. Sci. Rep. 2023, 13, 12533. https://doi.org/10.1038/s41598-023-39859-z.
- 26.Tejavath, V.; Kasarabada, V.; Gonuguntla, S.; et al. Technoeconomic investigation of Amine-Grafted zeolites and their kinetics for CO2 capture. ACS Omega 2021, 6, 6153–6162. https://doi.org/10.1021/acsomega.0c05397.
- 27.Bae, D.; Park, H.; Kim, J.S.; et al. Hydrogen adsorption in organic ion-exchanged zeolites. J. Phys. Chem. Solids 2007, 69, 1152–1154. https://doi.org/10.1016/j.jpcs.2007.10.068.
- 28.Zhang, L.; Wulf, T.; Baum, F.; et al. Chemical affinity of AG-Exchanged zeolites for efficient hydrogen isotope separation. Inorg. Chem. 2022, 61, 9413–9420. https://doi.org/10.1021/acs.inorgchem.2c00028.
- 29.Kordala, N.; Wyszkowski, M. Zeolite properties, methods of synthesis, and selected applications. Molecules 2024, 29, 1069. https://doi.org/10.3390/molecules29051069.
- 30.Akyalcin, S.; Akyalcin, L.; Ertugrul, E. Modification of natural clinoptilolite zeolite to enhance its hydrogen adsorption capacity. Res. Chem. Intermed. 2024, 50, 1455–1473. https://doi.org/10.1007/s11164-023-05212-2.
- 31.Germain, J.; Fréchet, J.M.J.; Svec, F. Nanoporous polymers for hydrogen storage. Small 2009, 5, 1098–1111. https://doi.org/10.1002/smll.200801762.
- 32.Karka, S.; Kodukula, S.; Nandury, S.V.; et al. Polyethylenimine-Modified Zeolite 13X for CO2 Capture: Adsorption and Kinetic Studies. ACS Omega 2019, 4, 16441–16449. https://doi.org/10.1021/acsomega.9b02047.
- 33.Altiparmak, İ. Hydrogen Adsorption on Cu (I)-Exchanged Zeolites. Master’s Thesis, Middle East Technical University, Ankara, Türkiye, 2018.
- 34.Rowsell JL, C.; Yaghi, O.M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the Low-Pressure hydrogen adsorption properties of Metal−Organic frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. https://doi.org/10.1021/ja056639q.
- 35.Bettahar, M.M. The Hydrogen Spillover Effect—A Misunderstanding Study II: Single Oxide and Zeolite Supports. Catalysts 2024, 14, 458. https://doi.org/10.3390/catal14070458 96.
- 36.Weitkamp, J.; Fritz, M.; Ernst, S. Zeolites as Media for Hydrogen Storage; Elsevier: Amsterdam, The Netherlands; 1993, pp. 11–19. https://doi.org/10.1016/b978-1-4832-8383-8.50086-6.
- 37.Öztürk, Z.; Köse, D.A.; Öztürk, B.; et al. Bazı Metal Organik kafes Yapılı Bileşiklerin Hidrojen Depolama Performanslarının İncelenmesi. 27 February 2014. Available online: https://dergipark.org.tr/tr/pub/gazimmfd/issue/6703/89091?utm_source=chatgpt.com (accessed on 21 December 2024).
- 38.Zhou, L.; Zhou, Y.; Sun, Y. Studies on the mechanism and capacity of hydrogen uptake by physisorption-based materials. Int. J. Hydrogen Energy 2005, 31, 259–264. https://doi.org/10.1016/j.ijhydene.2005.04.048.
- 39.Sharma, R.; Segato, T.; Delplancke, M.; et al. Hydrogen chloride removal from hydrogen gas by adsorption on hydrated ion-exchanged zeolites. Chem. Eng. J. 2019, 381, 122512. https://doi.org/10.1016/j.cej.2019.122512.
- 40.Li, Y.; Li, L.; Yu, J. Applications of zeolites in sustainable chemistry. Chem 2017, 3, 928–949. https://doi.org/10.1016/j.chempr.2017.10.009.
- 41.Aziz, M.T.; Naqvi SA, R.; Janjua, M.R.S.A.; et al. Exploring the adsorption behavior of molecular hydrogen on CHA-zeolite by comparing the performance of various force field methods. RSC Adv. 2023, 13, 30937–30950. https://doi.org/10.1039/d3ra04262f.
- 42.Raj, M.C.; Prasanth, K.P.; Dangi, G.P.; et al. Hydrogen sorption in transition metal exchanged zeolite Y: Volumetric measurements and simulation study. J. Porous Mater. 2011, 19, 657–666. https://doi.org/10.1007/s10934-011-9517-2.
- 43.Han, B.; Lv, P.; Sun, W.; et al. First-principles study on hydrogen storage performance of transition metal-doped zeolite template carbon. Crystals 2019, 9, 397. https://doi.org/10.3390/cryst9080397.
- 44.Areán, C.O.; Manoilova, O.; Bonelli, B.; et al. Thermodynamics of hydrogen adsorption on the zeolite Li-ZSM-5. Chem. Phys. Lett. 2003, 370, 631–635. https://doi.org/10.1016/s0009-2614(03)00172-6.
- 45.Zhang, Z.; Zhang, W.; Chen, X.; et al. Adsorption of CO2 on Zeolite 13X and Activated Carbon with Higher Surface Area. Sep. Sci. Technol. 2010, 45, 710–719. https://doi.org/10.1080/01496390903571192.
- 46.Oren, A.; Kaya, A. Factors affecting adsorption characteristics of Zn2+ on two natural zeolites. J. Hazard. Mater. 2005, 131, 59–65. https://doi.org/10.1016/j.jhazmat.2005.09.027.
- 47.Özkırım, İ.; Yörükoğulları, E. Manisa-Gördes Doğal Zeolitinin (Klinoptilolit) Bet Izoterm Karakteristikleri. 15 December 2005. Available online: https://dergipark.org.tr/tr/pub/dpufbed/issue/36210/407840?utm_source=chatgpt.com (accessed on 20 December 2024).
- 48.Semrau, A.L.; Stanley, P.M.; Huber, D.; et al. Vectorial catalysis in surface-anchored nanometer-sized metal–organic frameworks-based microfluidic devices. Angew. Chem. Int. Ed. 2021, 61, e202115100. https://doi.org/10.1002/anie.202115100.
- 49.Ren, J.; Langmi, H.W.; North, B.C.; et al. Review on processing of metal-organic framework (MOF) materials towards system integration for hydrogen storage. Int. J. Energy Res. 2014, 39, 607–620. https://doi.org/10.1002/er.3255.
- 50.Shet, S.P.; Priya, S.S.; Sudhakar, K.; et al. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. https://doi.org/10.1016/j.ijhydene.2021.01.020.
- 51.Mon, M.; Bruno, R.; Ferrando-Soria, J.; et al. Metal–organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947. https://doi.org/10.1039/c8ta00264a.
- 52.Langmi, H.W.; Ren, J.; North, B.; et al. Hydrogen storage in metal-organic frameworks: A review. Electrochim. Acta 2013, 128, 368–392. https://doi.org/10.1016/j.electacta.2013.10.190.
- 53.Li, J.; Cheng, S.; Zhao, Q.; et al. Synthesis and hydrogen-storage behavior of metal–organic framework MOF-5. Int. J. Hydrogen Energy 2008, 34, 1377–1382. https://doi.org/10.1016/j.ijhydene.2008.11.048.
- 54.Yang, S.J.; Jung, H.; Kim, T.; et al. Effects of structural modifications on the hydrogen storage capacity of MOF-5. Int. J. Hydrogen Energy 2012, 37, 5777–5783. https://doi.org/10.1016/j.ijhydene.2011.12.163.
- 55.Hirscher, M.; Panella, B. Nanostructures with high surface area for hydrogen storage. J. Alloys Compd. 2005, 404–406, 399–401. https://doi.org/10.1016/j.jallcom.2004.11.109.
- 56.Liu, S.; Zhang, C.; Sun, Y.; et al. Design of metal-organic framework-based photocatalysts for hydrogen generation. Coord. Chem. Rev. 2020, 413, 213266. https://doi.org/10.1016/j.ccr.2020.213266.
- 57.Han, S.S.; Deng, W.; Goddard, W.A. Improved designs of metal–organic frameworks for hydrogen storage. Angew. Chem. 2007, 119, 6405–6408. https://doi.org/10.1002/ange.200700303.
- 58.Suh, M.P.; Park, H.J.; Prasad, T.K.; et al. Hydrogen storage in metal–organic frameworks. Chem. Rev. 2011, 112, 782–835. https://doi.org/10.1021/cr200274s.
- 59.Park, J.; Adhikary, A.; Moon, H.R. Progress in the development of flexible metal–organic frameworks for hydrogen storage and selective separation of its isotopes. Coord. Chem. Rev. 2023, 497, 215402. https://doi.org/10.1016/j.ccr.2023.215402.
- 60.Tao, Y.; Xu, H. A critical review on potential applications of metal-organic frameworks (MOFs) in adsorptive carbon capture technologies. Appl. Therm. Eng. 2023, 236, 121504. https://doi.org/10.1016/j.applthermaleng.2023.121504.
- 61.Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2011, 112, 933–969. https://doi.org/10.1021/cr200304e.
- 62.Ding, M.; Cai, X.; Jiang, H. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. https://doi.org/10.1039/c9sc03916c.
- 63.Butova, V.V.; Soldatov, M.A.; Guda, A.A.; et al. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2015, 85, 280–307. https://doi.org/10.1070/rcr4554.
- 64.Yuan, S.; Feng, L.; Wang, K.; et al. Stable metal–organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. https://doi.org/10.1002/adma.201704303.
- 65.Liu, C.; Chen, Y.; Wu, C.; et al. Hydrogen storage in carbon nanotubes revisited. Carbon 2010, 48, 452–455. https://doi.org/10.1016/j.carbon.2009.09.060
- 66.Franco, A.; Giovannini, C. Hydrogen gas compression for efficient storage: Balancing energy and increasing density. Hydrogen 2024, 5, 293–311. https://doi.org/10.3390/hydrogen5020017.
- 67.Li, Y.; Yang, R.T. Hydrogen storage in low silica type X zeolites. J. Phys. Chem. B 2006, 110, 17175–17181. https://doi.org/10.1021/jp0634508.
- 68.Musyoka, N.M.; Ren, J.; Langmi, H.W.; et al. A comparison of hydrogen storage capacity of commercial and fly ash-derived zeolite X together with their respective templated carbon derivatives. Int. J. Hydrogen Energy 2015, 40, 12705–12712. https://doi.org/10.1016/j.ijhydene.2015.07.085.
- 69.Xu, Y.; Yang, X.; Li, Y.; et al. Rare-earth metal-based materials for hydrogen storage: Progress, challenges, and future perspectives. Nanomaterials 2024, 14, 1671. https://doi.org/10.3390/nano14201671.
- 70.Warahena, T.A. Applications of Zeolites for Sustainability—A Mini Review. NERS 2022, 2022, 136.
- 71.Möller, K.; Bein, T. Pores within pores—How to craft ordered hierarchical zeolites. Science 2011, 333, 297–298. https://doi.org/10.1126/science.1208528.
- 72.De Gennaro, B. Surface modification of zeolites for environmental applications In Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 57–85. https://doi.org/10.1016/b978-0-12-814617-0.00009-8.
- 73.Bonenfant, D.; Kharoune, M.; Niquette, P.; et al. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 2008, 9, 013007. https://doi.org/10.1088/1468-6996/9/1/013007.
- 74.Walton, K.S.; Abney, M.B.; LeVan, M.D. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. https://doi.org/10.1016/j.micromeso.2005.11.023.
- 75.Lutz, W. Zeolite Y: Synthesis, modification, and properties—A case revisited. Adv. Mater. Sci. Eng. 2014, 2014, 724248. https://doi.org/10.1155/2014/724248.
- 76.Zhang, B.; Liu, R.; Kimura, H.; et al. Phase transformation and performance of MG-Based hydrogen storage material by adding ZNO nanoparticles. Nanomaterials 2023, 13, 1321. https://doi.org/10.3390/nano13081321.
- 77.Bahmanzadegan, F.; Ghaemi, A. Modification and functionalization of zeolites to improve the efficiency of CO2 adsorption: A review. Case Stud. Chem. Environ. Eng. 2023, 9, 100564. https://doi.org/10.1016/j.cscee.2023.100564.
- 78.Tahraoui, Z.; Nouali, H.; Marichal, C.; et al. Zeolite-Polymer composite materials as water scavenger. Molecules 2021, 26, 4815. https://doi.org/10.3390/molecules26164815.
- 79.Bhattacharjee, S.; Jang, M.; Kwon, H.; et al. Zeolitic Imidazolate frameworks: Synthesis, functionalization, and Catalytic/Adsorption applications. Catal. Surv. Asia 2014, 18, 101–127. https://doi.org/10.1007/s10563-014-9169-8.
- 80.Somo, T.R.; Maponya, T.C.; Davids, M.W.; et al. A comprehensive review on hydrogen absorption behaviour of metal alloys prepared through mechanical alloying. Metals 2020, 10, 562. https://doi.org/10.3390/met10050562.
- 81.Rasul, M.; Hazrat, M.; Sattar, M.; et al. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. https://doi.org/10.1016/j.enconman.2022.116326.
- 82.Langmi, H.; Book, D.; Walton, A.; et al. Hydrogen storage in ion-exchanged zeolites. J. Alloys Compd. 2005, 404–406, 637–642. https://doi.org/10.1016/j.jallcom.2004.12.193.
- 83.Aceves, S.M.; Espinosa-Loza, F.; Ledesma-Orozco, E.; et al. High-density automotive hydrogen storage with cryogenic capable pressure vessels. Int. J. Hydrogen Energy 2009, 35, 1219–1226. https://doi.org/10.1016/j.ijhydene.2009.11.069.
- 84.Wang, H.; Gao, Q.; Hu, J.; et al. High performance of nanoporous carbon in cryogenic hydrogen storage and electrochemical capacitance. Carbon 2009, 47, 2259–2268. https://doi.org/10.1016/j.carbon.2009.04.021.
- 85.Rybár, P.; Drebenstedt, C.; Molokáč; M; et al. Storage of liquid hydrogen in natural zeolite. Acta Montan. Slovaca 2015, 20, 242–250.
- 86.Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. https://doi.org/10.1070/rcr5014.
- 87.Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. https://doi.org/10.1016/j.ijhydene.2020.05.021.
- 88.Qiu, Y.; Yang, H.; Tong, L.; et al. Research progress of cryogenic materials for storage and transportation of liquid hydrogen. Metals 2021, 11, 1101. https://doi.org/10.3390/met11071101.
- 89.Calleja, G.; Botas, J.; Sánchez-Sánchez, M.; et al. Hydrogen adsorption over Zeolite-like MOF materials modified by ion exchange. Int. J. Hydrogen Energy 2010, 35, 9916–9923. https://doi.org/10.1016/j.ijhydene.2010.02.114.
- 90.Deng, Q.; Elbeshbishy, E.; Lee, H. Simultaneous regeneration of exhausted zeolite and nitrogen recovery using an air stripping method at alkaline pH. Water Qual. Res. J. 2016, 51, 321–330. https://doi.org/10.2166/wqrjc.2016.007.
- 91.Ezzi, A.A.; Ismael, L.; Fayad, M.A.; et al. Experimental investigation of dehumidification and regeneration of zeolite coated energy exchanger. Int. J. Thermofluids 2022, 15, 100164. https://doi.org/10.1016/j.ijft.2022.100164.
- 92.Hassan, I.; Ramadan, H.S.M.; Saleh, M.; et al. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311.
- 93.Manda, T.; Barasa, G.O.; Louis, H.; et al. A data-guided approach for the evaluation of zeolites for hydrogen storage with the aid of molecular simulations. J. Mol. Model. 2024, 30, 43. https://doi.org/10.1007/s00894-024-05837-z.
- 94.Musyoka, N.M.; Ren, J.; Annamalai, P.; et al. Synthesis of a hybrid MIL-101(Cr)/ZTC composite for hydrogen storage applications. Res. Chem. Intermed. 2015, 42, 5299–5307. https://doi.org/10.1007/s11164-015-2361-2.
- 95.Liu, M.; Miao, C.; Wu, Z. Recent advances in the synthesis, characterization, and catalytic consequence of metal species confined within zeolite for hydrogen-related reactions. Ind. Chem. Mater. 2023, 2, 57–84. https://doi.org/10.1039/d3im00074e.
- 96.Kim, J.; Kim, K.D.; Jung, U.; et al. Clean hydrogen production from ammonia decomposition over zeolite 13X-supported Ni catalysts. Sustain. Energy Fuels 2024, 8, 896–904. https://doi.org/10.1039/d3se01426f.
How to Cite
Taşğın, B.; Ryšavý, J.; Sangeetha, T.; Yan, W.-M. Hydrogen Storage in Zeolites: A Mini Review of Structural and Chemical Influences on Adsorption Performance. Green Energy and Fuel Research 2025, 2 (1), 48–63. https://doi.org/10.53941/gefr.2025.100005.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


