Catalytic pyrolysis has emerged as a pivotal technology for converting renewable diverse feedstocks (i.e., lignocellulosic biomass, algal biomass, and plastic wastes) into biofuels and chemicals. This review comprehensively examines the reaction kinetics in catalytic pyrolysis, addressing the fundamental gap between lab-scale research and industrial applications. The mechanisms of conventional (i.e., electrical heating) and microwave-assisted catalytic pyrolysis are detailed, highlighting the role of catalysts in altering reaction rates, reaction pathways, and decreasing activation energies. This paper delves into kinetic analysis techniques by comparing the model-free and model-fitting approaches and exploring the emerging role of machine learning in predicting kinetic parameters. In addition, it extensively explores the feedstock specific kinetic models, highlighting the behavior of pseudo-components of lignocellulosic feedstocks, plastic wastes, and their mixtures with a specific focus on synergistic effect during co-pyrolysis. Further, an essential framework to integrate molecular-scale phenomena with reactor-scale process performance was presented by exploring the advanced modelling techniques such as microkinetic modelling using density functional theory (DFT), lumped system analysis using process simulations, and catalyst deactivation kinetics. Despite its promise, challenges such as catalyst deactivation, heat and mass transfer limitations, and feedstock variability remain critical hurdles. This review concludes by identifying future research directions, emphasizing the in-situ characterization, integration of machine learning and artificial intelligence for process optimization, and kinetics of emerging catalyst systems to facilitate the commercial deployment of predictive models for catalytic pyrolysis technologies.
- Open Access
- Review
Kinetics for Catalytic Pyrolysis of Organic Solid Wastes
- Shri Ram 1,
- Yogesh Patil 2,
- Fatma Abdelrhman 1,3,
- Tarique Ahmed Memon 4,
- Yaning Zhang 1,*
Author Information
Received: 24 Nov 2025 | Revised: 26 Dec 2025 | Accepted: 26 Dec 2025 | Published: 30 Dec 2025
Abstract
Graphical Abstract
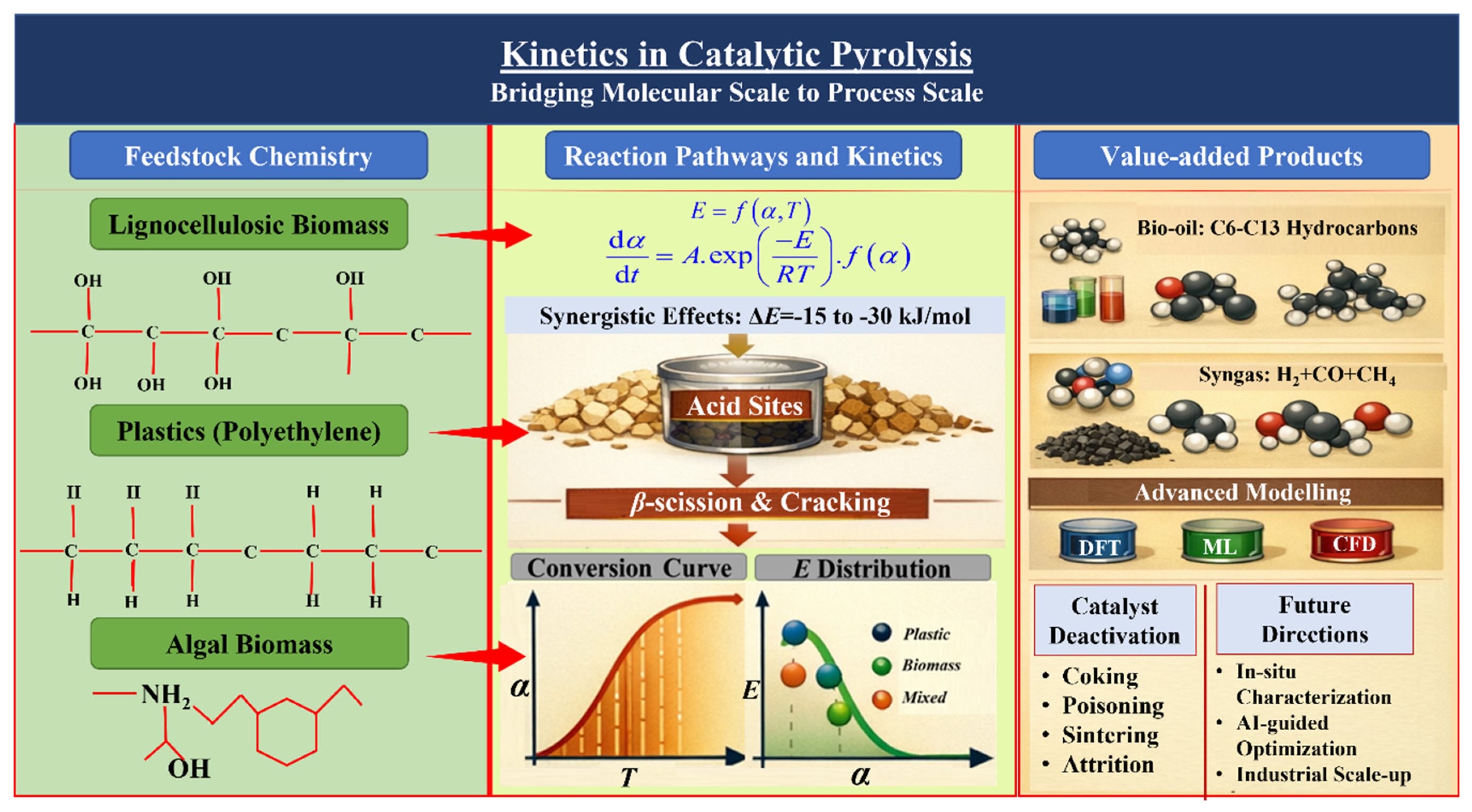
Keywords
pyrolysis | catalyst | kinetics | plastic waste | biomass
References
- 1.
Hsiao, C.Y.; Lin, S.L. Thermal Reactions and Byproducts from the Waste-to-Energy Process of Flame Retardant-Containing Wastes-A Review. Green Energy Fuel Res. 2025, 2, 109–126. https://doi.org/10.53941/gefr.2025.100009.
- 2.
Marwan-Abdelbaset, E.; Lu, X.Y.; Samy-Kamal, M.; et al. From waste to value: Microbial valorization of low-cost renewable resources for hyaluronic acid production. Waste Manag. 2026, 209, 115179. https://doi.org/10.1016/j.wasman.2025.115179.
- 3.
Ajona, C.; Saravanakumar, A. Analysis of Thermal Properties in Co-Gasification of Municipal Solid Waste and Woody Biomass. Green Energy Fuel Res. 2025, 2, 139–151. https://doi.org/10.53941/gefr.2025.100011.
- 4.
Melikoglu, M. Waste valorization strategies with inputs for microalgae biorefineries: A global review. Clean. Water 2025, 4, 100125. https://doi.org/10.1016/j.clwat.2025.100125.
- 5.
Yesil, H.; Tugtas, A.E.; Çalli, B. Beyond landfills: Transforming biodegradable waste into climate solutions and valuable resources. Rev. Environ. Sci. Bio Technol. 2025, 24, 805–829. https://doi.org/10.1007/s11157-025-09739-1.
- 6.
Fang, Y.; Tang, Y.; Li, G.; et al. Co-pyrolysis of waste biomass and plastics from food packaging waste: Pyrolysis characteristics, synergetic effects, and reaction kinetics. J. Environ. Manag. 2025, 395, 127800. https://doi.org/10.1016/j.jenvman.2025.127800.
- 7.
Sun, Y.; Deng, J.; Zhang, F.; et al. Global distribution and warming effect of brown carbon from shipping emissions. Carbon Res. 2025, 4, 44. https://doi.org/10.1007/s44246-025-00212-w.
- 8.
Sun, D.; Zeng, X.; Wang, F.; et al. Gas generation characteristics and kinetics during catalytic pyrolysis of distiller’s grains by coal slag for coordinated utilization via gasification. J. Environ. Manag. 2025, 394, 127388. https://doi.org/10.1016/j.jenvman.2025.127388.
- 9.
Yoon, S.; Lee, Y.; An, H.; et al. Sustainable woody biochar application for improving net ecosystem carbon budget, yield and soil properties in red pepper cropping systems: A two-year field study. Biochar 2025, 7, 112. https://doi.org/10.1007/s42773-025-00494-8.
- 10.
Ramandani, A.A.; Rachmadona, N.; Munawaroh, H.S.H.; et al. Sustaining Food Waste for Energy Conversion: A Mini Review. Green Energy Fuel Res. 2025, 2, 34–47. https://doi.org/10.53941/gefr.2025.100004.
- 11.
Vasudev, V.; Ku, X.; Lin, J. Kinetic study and pyrolysis characteristics of algal and lignocellulosic biomasses. Bioresour. Technol. 2019, 288, 121496. https://doi.org/10.1016/j.biortech.2019.121496.
- 12.
Lee, D.; Nam, H.; Seo, M.W.; et al. Recent progress in the catalytic thermochemical conversion process of biomass for biofuels. Chem. Eng. J. 2022 447, 137501. https://doi.org/10.1016/j.cej.2022.137501.
- 13.
El-Fawal, E.M.; El-Naggar, A.M.A.; El-Zahhar, A.A.; et al. Biofuel production from waste residuals: Comprehensive insights into biomass conversion technologies and engineered biochar applications. RSC Adv. 2025, 15, 11942–11974. https://doi.org/10.1039/D5RA00857C.
- 14.
Wang, W.; Gu, Y.; Zhou, C.; et al. Current Challenges and Perspectives for the Catalytic Pyrolysis of Lignocellulosic Biomass to High-Value Products. Catalysts 2022, 12, 1524. https://doi.org/10.3390/catal12121524.
- 15.
El-Araby, R. Biofuel production: Exploring renewable energy solutions for a greener future. Biofuels Bioprod. Biorefin. 2024, 17, 129. https://doi.org/10.1186/s13068-024-02571-9.
- 16.
Ram, S.; Ku, X.; Vasudev, V. Catalytic pyrolysis of lignocellulosic and algal biomass using NaOH as a catalyst. Biofuels Bioprod. Biorefin. 2024, 18, 482–494. https://doi.org/10.1002/bbb.2599.
- 17.
Sulis, D.B.; Lavoine, N.; Sederoff, H.; et al. Advances in lignocellulosic feedstocks for bioenergy and bioproducts. Nat. Commun. 2025, 16, 1244. https://doi.org/10.1038/s41467-025-56472-y.
- 18.
Nosenzo, S.; Kelman, R. From fields to fuel: Analyzing the global economic and emissions potential of agricultural pellets, informed by a case study. arXiv 2025, arXiv:2508.12457.
- 19.
Loc, N.X.; Phuong, D.T.M. Optimizing biochar production: A review of recent progress in lignocellulosic biomass pyrolysis. Front. Agric. Sci. Eng. 2025, 12, 148–172. https://doi.org/10.15302/J-FASE-2024597.
- 20.
Ziolkowska, J.R. Biofuels technologies: An overview of feedstocks, processes, and technologies. Biofuels A More Sustain. Future 2019, 1–19. https://doi.org/10.1016/B978-0-12-815581-3.00001-4.
- 21.
Selwal, N.; Sultana, H.; Rahayu, F.; et al. Emerging technologies in biomass conversion: Bioengineering and nanocatalysts to AI-driven process optimization. Biomass Bioenergy 2025, 200, 108054. https://doi.org/10.1016/j.biombioe.2025.108054.
- 22.
Ram, S.; Ku, X.; Vasudev, V.; et al. Pyrolytic performance and kinetic analysis of non-catalytic and catalytic pyrolysis of bamboo powder and red algae. Biomass Convers. Biorefin. 2025, 15, 25117–25129. https://doi.org/10.1007/s13399-025-06810-3.
- 23.
Ram, S.; Yadav, S.K.; Yadav, A.; et al. Recent Advancements in Thermochemical Conversion of Biomass and Technologies Used to Eliminate the Tar Formation. In Biennial International Conference on Future Learning Aspects of Mechanical Engineering; Springer Nature Singapore: Singapore, 2023; pp. 585–599. https://doi.org/10.1007/978-981-99-2382-3_49.
- 24.
Wei, F.; Sang, S.; Liu, S.; et al. BECCS carbon-negative technologies based on biomass thermochemical conversion: A review of critical pathways and research advances. Fuel 2025, 390, 134743. https://doi.org/10.1016/j.fuel.2025.134743.
- 25.
Martínez, J.D.; Puy, N.; Murillo, R.; et al. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. https://doi.org/10.1016/j.rser.2013.02.038.
- 26.
Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Process. Technol. 2018, 180, 32–46. https://doi.org/10.1016/j.fuproc.2018.08.002.
- 27.
Patlolla, S.R.; Katsu, K.; Sharafian, A.; et al. A review of methane pyrolysis technologies for hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113323. https://doi.org/10.1016/j.rser.2023.113323.
- 28.
Chanda, M. Chemical aspects of polymer recycling. Adv. Ind. Eng. Polym. Res. 2021, 4, 133–150. https://doi.org/10.1016/j.aiepr.2021.06.002.
- 29.
Chen, Z.; Wei, W.; Chen, X.; et al. Upcycling of plastic wastes for hydrogen production: Advances and perspectives. Renew. Sustain. Energy Rev. 2024, 195, 114333. https://doi.org/10.1016/j.rser.2024.114333.
- 30.
Shoaib, M.; Ku, X.; Ram, S. Thermogravimetric characteristics and product distribution behavior during co-pyrolysis of Datong coal with two different biomasses. Int. J. Coal Prep. Util. 2025, 1–16. https://doi.org/10.1080/19392699.2025.2563811.
- 31.
Aysu, T.; Durak, H. Catalytic pyrolysis of liquorice (Glycyrrhiza glabra L.) in a fixed-bed reactor: Effects of pyrolysis parameters on product yields and character. J. Anal. Appl. Pyrolysis 2015, 111, 156–172. https://doi.org/10.1016/j.jaap.2014.11.017.
- 32.
Ismail, T.M.; Banks, S.W.; Yang, Y.; et al. Coal and biomass co-pyrolysis in a fluidized-bed reactor: Numerical assessment of fuel type and blending conditions. Fuel 2020, 275, 118004. https://doi.org/10.1016/j.fuel.2020.118004.
- 33.
Wu, Y.; Wang, H.; Li, H.; et al. Applications of catalysts in thermochemical conversion of biomass (pyrolysis, hydrothermal liquefaction and gasification): A critical review. Renew. Energy 2022, 196, 462–481. https://doi.org/10.1016/j.renene.2022.07.031.
- 34.
Yani, A.; Wijayanti, W.; Sasongko, M.N.; et al. Synergistic co-pyrolysis of polyethylene terephthalate and Hibiscus rosa-sinensis: Catalytic mechanisms for optimizing pyrolysis yields. South Afr. J. Chem. Eng. 2025, 53, 281–302. https://doi.org/10.1016/j.sajce.2025.05.008.
- 35.
He, Q.; Wang, Q.; Luan, J. A review on biomass pyrolysis and pyrolysis mechanisms. Biofuels 2025, 1–21. https://doi.org/10.1080/17597269.2025.2537515.
- 36.
Kim, J.Y.; Yoo, Y.; Lee, D.J.; et al. Catalytic pyrolysis of three industrial fungal biomass to enhance syngas production. Fuel 2026, 405, 136748. https://doi.org/10.1016/j.fuel.2025.136748.
- 37.
Tahir, M.H.; Ali, A.; Vasudev, V. Enhancing monocyclic aromatics hydrocarbons production via catalytic co-pyrolysis of cabbage biomass and low-density polyethylene with ZnO/ZSM-5 catalyst. J. Anal. Appl. Pyrolysis 2026, 193, 107391. https://doi.org/10.1016/j.jaap.2025.107391.
- 38.
Raj, A.; Ghodke, P.K. Investigation of in situ and ex situ catalytic pyrolysis of lignocellulosic biomass waste for the production of de-oxygenated C8–C18 range enhanced liquid fuel precursor—reaction mechanisms and optimization. J. Anal. Appl. Pyrolysis 2025, 191, 107179. https://doi.org/10.1016/j.jaap.2025.107179.
- 39.
Xie, K.; Liu, Z.; Zhang, M.; et al. Understanding the Transient Microwave Drying Performances of Industrial Sewage Sludge Towards Green Fuel and Energy. Green Energy Fuel Res. 2025, 2, 174–186. https://doi.org/10.53941/gefr.2025.100013.
- 40.
Zhao, W.; Cao, W.; Cui, L.; et al. Energy and Exergy Performances of Corn Straw and SiC during the Microwave Heating Process. J. Therm. Sci. 2025, 34, 1857–1866. https://doi.org/10.1007/s11630-025-2173-7.
- 41.
Ke, L.; Zhou, N.; Wu, Q.; et al. Microwave catalytic pyrolysis of biomass: A review focusing on absorbents and catalysts. NPJ Mater. Sustain. 2024, 2, 24. https://doi.org/10.1038/s44296-024-00027-7.
- 42.
Wei, S.; Zhou, T.; Yang, D.; et al. Microwave-induced and catalytic pyrolysis of municipal domestic waste under multiple synergistic effects. J. Renew. Sustain. Energy 2025, 17, 023101. https://doi.org/10.1063/5.0250134.
- 43.
Tiwari, M.; Vinu, R. In situ and ex situ catalytic microwave pyrolysis of biomass pellets using Ni/Al2O3 for hydrogen and bio-oil production. J. Anal. Appl. Pyrolysis 2025, 189, 107044. https://doi.org/10.1016/j.jaap.2025.107044.
- 44.
Yang, X.; Yu, J.; Zeng, M.; et al. Complete valorization of lignocellulosic biomass through integrated reductive catalytic fractionation and microwave-assisted pyrolysis. J. Anal. Appl. Pyrolysis 2025, 188, 107049. https://doi.org/10.1016/j.jaap.2025.107049.
- 45.
Shi, X.; Wang, B.; Hu, J.; et al. Investigating the synergistic driving action of microwave and char-based multi-catalysts on biomass catalytic pyrolysis into value-added bio-products. Renew. Energy 2023, 219, 119490. https://doi.org/10.1016/j.renene.2023.119490.
- 46.
He, M.; Zhao, J.; Wang, D.; et al. Microwave-assisted catalytic pyrolysis of biomass with biochar materials derived from spent lithium-ion batteries: Microwave absorption and pyrolysis characteristics. J. Environ. Chem. Eng. 2024, 12, 112099. https://doi.org/10.1016/j.jece.2024.112099.
- 47.
Sharma, S.; Tsai, M.L.; Sharma, V.; et al. Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2023, 10, 6. https://doi.org/10.3390/environments10010006.
- 48.
Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. https://doi.org/10.1016/j.biortech.2022.128087.
- 49.
Zhang, J.; Zhang, B.; Xia, A.; et al. Production of carbon dots, biofuels, bio-adsorbents, and biological nutrients via hydrothermal conversion of Chlorella pyrenoidosa and oilseed rape straw. Biochar 2025, 7, 109. https://doi.org/10.1007/s42773-025-00482-y.
- 50.
Du, K.; Zhong, W.; Chen, X.; et al. Co-Pyrolysis of Coal and Waste Plastic: Characterization and Rapid Tar Yield Prediction Method. J. Therm. Sci. 2025, 34, 1599–1611. https://doi.org/10.1007/s11630-025-2174-6.
- 51.
Islam, K.M.O.; Ahmad, N.; Ummer, A.C.; et al. Microwave-Assisted pyrolysis of waste plastics: A comprehensive review on process parameters, catalysts, and future prospects. Results Eng. 2025, 26, 105571. https://doi.org/10.1016/j.rineng.2025.105571.
- 52.
Yuan, J.; Yang, G.; Zhou, X.; et al. Functional carbon materials from waste plastics: Synthesis and applications. Sustain. Carbon Mater. 2025, 1, e002. https://doi.org/10.48130/scm-0025-0005.
- 53.
Huang, C.; Tang, C.; Liu, J.; et al. Insight into the catalytic role of industrial solid waste in improving gas quality during biomass pyrolysis. Carbon Resour. Convers. 2025, 100339. https://doi.org/10.1016/j.crcon.2025.100339.
- 54.
Zhu, Z.; Zhou, Z.; Zhang, Z.; et al. Analysis of municipal solid waste (MSW) pyrolysis products using synchrotron vacuum ultraviolet photoionization mass spectrometry: Effects of CaO and NH3. J. Anal. Appl. Pyrolysis 2026, 193, 107441. https://doi.org/10.1016/j.jaap.2025.107441.
- 55.
Murphy, F.; Gusciute, E.; Mediboyina, K.; et al. Social and Environmental Sustainability of Municipal Solid Waste in the Context of the UN Sustainable Development Goals; IEA Bioenergy: Ottawa, ON, Canada, 2025.
- 56.
Suriapparao, D.V.; Sridevi, V.; Ramesh, P.; et al. Synthesis of sustainable chemicals from waste tea powder and Polystyrene via Microwave-Assisted in-situ catalytic Co-Pyrolysis: Analysis of pyrolysis using experimental and modeling approaches. Bioresour. Technol. 2022, 362, 127813. https://doi.org/10.1016/j.biortech.2022.127813.
- 57.
Bakhtiar, M.; Shahbaz, M.; Ayub, H.M.U.; et al. Steam gasification of plastic and woody biomass for hydrogen-rich syngas production with CO2 reduction, emission and energy analysis. Energy 2025, 334, 137718. https://doi.org/10.1016/j.energy.2025.137718.
- 58.
Liu, M.; Dong, W.; Ni, H.; et al. Co-pyrolysis of sea rice straw and low-density polypropylene: Implications for advancing sustainable fuel production. J. Anal. Appl. Pyrolysis 2025, 192, 107283. https://doi.org/10.1016/j.jaap.2025.107283.
- 59.
Li, X.; Chen, X.; Zhang, C.; et al. PM10 emissions from co-combustion of water washed sea rice waste with coal. Appl. Energy 2024, 356, 122446. https://doi.org/10.1016/j.apenergy.2023.122446.
- 60.
Sathish, T.; Ammar, M.B.; Sabeur, H.; et al. Hydrogen production from greywater algae biomass via Pyrolysis: Influence of temperature and polyaniline integrated sodium hydroxide (PANI-NaOH) catalyst. Case Stud. Therm. Eng. 2025, 73, 106639. https://doi.org/10.1016/j.csite.2025.106639.
- 61.
Ong, M.Y.; Milano, J.; Nomanbhay, S.; et al. Insights into algae-plastic pyrolysis: Thermogravimetric and kinetic approaches for renewable energy. Energy 2025, 314, 134322. https://doi.org/10.1016/j.energy.2024.134322.
- 62.
Zhao, Z.; Tian, H.; Chen, Z.; et al. Upgrading of bio-oil from torrefied wheat straw over Fe-Ni modified HZSM-5@MCM-41: Influence of pyrolysis temperature and catalyst-feedstock ratio. J. Energy Inst. 2026, 124, 102366. https://doi.org/10.1016/j.joei.2025.102366.
- 63.
Patil, Y.; Ku, X. Pyrolysis kinetics and thermodynamic behavior of pseudo components of raw and torrefied maple wood. Energy Sources Part A 2024, 46, 462–474. https://doi.org/10.1080/15567036.2023.2285406.
- 64.
State, R.N.; Ionescu, G.; Volceanov, A.; et al. Combined valorization of bone waste as feedstock and support material for ex-situ catalytic pyrolysis. J. Environ. Chem. Eng. 2025, 13, 119091. https://doi.org/10.1016/j.jece.2025.119091.
- 65.
Patil, Y.; Ku, X. Comparison and characterization of torrefaction performance and pyrolysis behaviour of softwood and hardwood. Energy Sources Part A 2022, 44, 8860–8877. https://doi.org/10.1080/15567036.2022.2126561.
- 66.
Isahak, W.N.R.W.; Al-Amiery, A. Catalysts driving efficiency and innovation in thermal reactions: A comprehensive review. Green Technol. Sustain. 2024, 2, 100078. https://doi.org/10.1016/j.grets.2024.100078.
- 67.
Hussain, A.; Ghaffar, I.; Sattar, S.; et al. Eco-friendly Catalysts Revolutionizing Energy and Environmental Applications: An Overview. Top. Catal. 2025, 68, 487–509. https://doi.org/10.1007/s11244-024-01976-y.
- 68.
Shanthini, V.S.; Chitra, D.; Moorthy, G. Biodiesel: A comprehensive review of properties, catalyst types, and feedstock sources. Results Chem. 2025, 18, 102678. https://doi.org/10.1016/j.rechem.2025.102678.
- 69.
Kojima, T. Chapter 1 Overview of the Catalytic Chemistry of Metal Complexes. R. Soc. Chem. 2024, 2, 1–7. https://doi.org/10.1039/9781837676484-00001.
- 70.
Mirshafiee, F.; Khoshbin, R.; Karimzadeh, R. A green approach for template free synthesis of Beta zeolite incorporated in ZSM-5 zeolite to enhance catalytic activity in MTG reaction: Effect of seed nature and temperature. J. Clean. Prod. 2022, 361, 132159. https://doi.org/10.1016/j.jclepro.2022.132159.
- 71.
Yoshimura, T.; Tanaka, S.; Matsuda, N.; et al. Estimation of catalytic cracking of vacuum gas oil by ZSM-5- and β-zeolite-containing two-layered and novel three-layered hierarchical catalysts using Curie point pyrolyzer. J. Anal. Appl. Pyrolysis 2024, 182, 106621. https://doi.org/10.1016/j.jaap.2024.106621.
- 72.
Pacheco, J.G.A.; Padilha, J.F.; Santos, B.L.; et al. Hydrogen-free deoxygenation of oleic acid on acidic and basic ZSM-5 and Y-zeolites: Products for biofuel and reaction pathways. Catal. Today 2025, 445, 115094. https://doi.org/10.1016/j.cattod.2024.115094.
- 73.
Li, H.; Zhang, Z.; Hu, J.; et al. Revealing the mechanisms of biomass fast pyrolysis catalyzed by CaO-modified red mud. J. Anal. Appl. Pyrolysis 2026, 193, 107444. https://doi.org/10.1016/j.jaap.2025.107444.
- 74.
Keller, M.H.; Moreira, R.; de Bona, J.; et al. Covalent triazine frameworks with MgO sites as a basic catalyst for aldol condensation and transesterification reactions. Appl. Catal. A Gen. 2025, 708, 120583. https://doi.org/10.1016/j.apcata.2025.120583.
- 75.
Cao, Z.; Niu, J.; Gu, Y.; et al. Catalytic pyrolysis of rice straw: Screening of various metal salts, metal basic oxide, acidic metal oxide and zeolite catalyst on products yield and characterization. J. Clean. Prod. 2020, 269, 122079. https://doi.org/10.1016/j.jclepro.2020.122079.
- 76.
Zhang, Z.; Zhang, X.; Zhang, L.; et al. Steam reforming of guaiacol over Ni/SiO2 catalyst modified with basic oxides: Impacts of alkalinity on properties of coke. Energy Convers. Manag. 2020, 205, 112301. https://doi.org/10.1016/j.enconman.2019.112301.
- 77.
Memarian, Z.; Meshkani, F. CO2 reforming of glycerol on Ni/Al2O3 catalyst: Influence of doping of alkaline earth metals (Mg, Ca, Sr, and Ba) to support. Biomass Bioenergy 2025, 193, 107578. https://doi.org/10.1016/j.biombioe.2024.107578.
- 78.
Zhang, Z.; Yu, M.; Shen, M.; et al. Promoting effect of alkaline earth metals on Ni/CeO2 catalysts for ammonia decomposition reaction. Mol. Catal. 2025, 578, 115016. https://doi.org/10.1016/j.mcat.2025.115016.
- 79.
Hemberger, P.; Custodis, V.B.F.; Bodi, A.; et al. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat. Commun. 2017, 8, 15946. https://doi.org/10.1038/ncomms15946.
- 80.
Chaihad, N.; Karnjanakom, S.; Abudula, A.; et al. Zeolite-based cracking catalysts for bio-oil upgrading: A critical review. Resour. Chem. Mater. 2022, 1, 167–183. https://doi.org/10.1016/j.recm.2022.03.002.
- 81.
Ye, R.; Ding, J.; Reina, T.R.; et al. Design of catalysts for selective CO2 hydrogenation. Nat. Synth. 2025, 4, 288–302. https://doi.org/10.1038/s44160-025-00747-1.
- 82.
Wang, G.; Dai, Y.; Yang, H.; et al. A review of recent advances in biomass pyrolysis. Energy Fuels 2020, 34, 15557–15578. https://doi.org/10.1021/acs.energyfuels.0c03107.
- 83.
Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Sikarwar, V.S.; et al. A critical review on biomass pyrolysis: Reaction mechanisms, process modeling and potential challenges. J. Energy Inst. 2023, 108, 101236. https://doi.org/10.1016/j.joei.2023.101236.
- 84.
Wang, S.; Dai, G.; Yang, H.; et al. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. https://doi.org/10.1016/j.pecs.2017.05.004.
- 85.
Huang, M.; Zhu, L.; Zhang, W.; et al. Insight into the synergistic reaction mechanism of biomass pseudo components and low-density polyethylene for the production of light aromatics through co-catalytic fast pyrolysis over hierarchical HZSM-5. Fuel 2022, 324, 124699. https://doi.org/10.1016/j.fuel.2022.124699.
- 86.
Razzak, S.A.; Haddad, H.; Nawaz, A.; et al. Review for the synergistic effects in catalytic co-pyrolysis of biomass and plastic waste: Pathways to sustainable energy. Next Sustainability 2025, 6, 100157. https://doi.org/10.1016/j.nxsust.2025.100157.
- 87.
Zhang, Y.; Wang, J.; Zhang, H.; et al. In-situ reaction strengthening mechanism of the pyrolysis water and bio-oil during the biomass pyrolysis over CaO and Ni-char dual catalytic system. Energy 2025, 319, 134913. https://doi.org/10.1016/j.energy.2025.134913.
- 88.
Hasan, M.; Baheerathan, B.; Sutradhar, S.; et al. Microwave-assisted synthesis of biomass-derived N-doped carbon dots for metal ion sensing. Carbon Res. 2025, 4, 49. https://doi.org/10.1007/s44246-025-00215-7.
- 89.
Imran, A.; Bramer, E.A.; Seshan, K.; et al. An overview of catalysts in biomass pyrolysis for production of biofuels. Biofuel 2018, 5, 872–882. https://doi.org/10.18331/BRJ2018.5.4.2.
- 90.
Norouzi, O.; Taghavi, S.; Arku, P.; et al. What is the best catalyst for biomass pyrolysis? J. Anal. Appl. Pyrolysis 2021, 158, 105280. https://doi.org/10.1016/j.jaap.2021.105280.
- 91.
Sun, M.H.; Chen, L.H.; Li, X.Y.; et al. A comparative study of hierarchically micro-meso-macroporous solid-acid catalysts constructed by zeolites nanocrystals synthesized via a quasi-solid-state crystallization process. Microporous Mesoporous Mater. 2013, 182, 122–135. https://doi.org/10.1016/j.micromeso.2013.08.034.
- 92.
Mustapha, S.I.; Isa, Y.M. Co-pyrolysis of microalgae and sewage sludge over ZnO/MgO/CeO2/HZSM-5 catalyst for energy and water treatment application. J. Environ. Chem. Eng. 2024, 12, 114955. https://doi.org/10.1016/j.jece.2024.114955.
- 93.
Kai, W.; Xie, X.; Lin, D.; et al. Catalytic pyrolysis of paper mill sludge over self-sourced CaO/biochar catalyst under different temperatures. Fuel 2025, 398, 135538. https://doi.org/10.1016/j.fuel.2025.135538.
- 94.
Zhou, Q.; Wu, L.; Wang, J.; et al. Catalytic fast pyrolysis of Ca(OH)2-pretreated lignin over Beta zeolite for aromatic-rich bio-oil production. Energy 2025, 328, 136667. https://doi.org/10.1016/j.energy.2025.136667.
- 95.
Rahimi, S.; Shahdadi, A.; Alizadeh, R.; et al. Conversion of polypropylene into valued-added gasoline range hydrocarbons by catalytic pyrolysis at atmospheric pressure: Different composite catalysts of HZSM-5, HY and MCM-41. J. Taiwan Inst. Chem. Eng. 2025, 173, 106184. https://doi.org/10.1016/j.jtice.2025.106184.
- 96.
Subagyono, R.D.J.N.; Madani, N.M.; Prechisilia, C.Z.L.C.B.; et al. Pyrolysis of microalgae over Ni/Al-SBA-15 and Ni/Ga-SBA-15 catalysts prepared using a low-acidity solvent and ultrasonic-assisted sol-gel method. J. Anal. Appl. Pyrolysis 2025, 186, 106935. https://doi.org/10.1016/j.jaap.2024.106935.
- 97.
Vichaphund, S.; Wimuktiwan, P.; Soongprasit, C.; et al. Aromatic and aliphatic production of catalytic pyrolysis of lignin using ZSM-5/Al-SBA-15 catalyst derived from high-calcium fly ash. Energy Rep. 2021, 7, 232–247. https://doi.org/10.1016/j.egyr.2021.07.127.
- 98.
Suarez, M.A.; Santamaria, L.; Lopez, G.; et al. Oxidative steam reforming of HDPE pyrolysis volatiles on Ni catalysts: Effect of the support (Al2O3, ZrO2, SiO2) and promoter (CeO2, La2O3) on the catalyst performance. Chin. J. Catal. 2025, 69, 149–162. https://doi.org/10.1016/S1872-2067(24)60222-6.
- 99.
Ma, C.; Kamo, T. Enhanced debromination by Fe particles during the catalytic pyrolysis of non-metallic fractions of printed circuit boards over ZSM-5 and Ni/SiO2-Al2O3 catalyst. J. Anal. Appl. Pyrolysis 2019, 138, 170–177. https://doi.org/10.1016/j.jaap.2018.12.021.
- 100.
Zheng, Y.; Tao, L.; Yang, X.; et al. Comparative study on pyrolysis and catalytic pyrolysis upgrading of biomass model compounds: Thermochemical behaviors, kinetics, and aromatic hydrocarbon formation. J. Energy Inst. 2019, 92, 1348–1363. https://doi.org/10.1016/j.joei.2018.09.006.
- 101.
Raja, N.; Monsalve-Bravo, G.M.; Kaneti, Y.V.; et al. Thermogravimetric kinetic analysis of catalytic and non-catalytic pyrolysis of simulated municipal solid waste. Chem. Eng. J. 2023, 470, 144046. https://doi.org/10.1016/j.cej.2023.144046.
- 102.
Dammann, M.; Walker, S.C.; Mancini, M.; et al. Devolatilisation of beech wood char: Kinetics from thermogravimetric analyses and drop-tube reactor experiments. Fuel 2024, 375, 131967. https://doi.org/10.1016/j.fuel.2024.131967.
- 103.
Alsaffar, M.A.; Mageed, A.K.; Ghany, M.A.R.A. Thermogravimetric analysis and non-isothermal kinetics of hydrocarbon-rich petroleum residues. Sustain. Chem. Clim. Action 2025, 7, 100115. https://doi.org/10.1016/j.scca.2025.100115.
- 104.
Memon, T.A.; Ku, X.; Vasudev, V.; et al. Experimental investigation of co-pyrolysis of fruit peel waste: Impact of blending on thermal degradation behavior, kinetics, and products. Biomass Convers. Biorefin. 2025, 15, 18783–18797. https://doi.org/10.1007/s13399-025-06550-4.
- 105.
Gamlin, C.D.; Dutta, N.K.; Choudhury, N.R.; et al. Evaluation of kinetic parameters of thermal and oxidative decomposition of base oils by conventional, isothermal and modulated TGA, and pressure DSC. Thermochim. Acta 2002, 392–393, 387–369. https://doi.org/10.1016/S0040-6031(02)00121-1.
- 106.
Chen, T.; Liang, A.; Chen, X.; et al. Pyrolysis behavior of polypropylene and PVC medical waste: Multi-scale analysis via TGA, FTIR, GC–MS and kinetic modeling. Therm. Sci. Eng. Prog. 2025, 104324. https://doi.org/10.1016/j.tsep.2025.104324.
- 107.
Shoaib, M.; Ku, X.; Vasudev, V. Thermo-kinetic analysis of co-pyrolysis of Platanus tree leaves with coals. Int. J. Coal Prep. Util. 2025, 68, 104324. https://doi.org/10.1080/19392699.2025.2480334.
- 108.
Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; et al. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. https://doi.org/10.1016/j.tca.2011.03.034.
- 109.
Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; et al. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. https://doi.org/10.1016/j.tca.2020.178597.
- 110.
Vasudev, V.; Ku, X.; Ram, S.; et al. An Exploration of Strategies for Conducting Kinetic Analysis of Lignocellulosic and Algal Biomass Pyrolysis. Bioenergy Res. 2025, 18, 64. https://doi.org/10.1007/s12155-025-10861-9.
- 111.
Patil, Y.; Ku, X.; Vasudev, V. Pyrolysis Characteristics and Determination of Kinetic and Thermodynamic Parameters of Raw and Torrefied Chinese Fir. ACS Omega 2023, 8, 34938–34947. https://doi.org/10.1021/acsomega.3c04328.
- 112.
Memon, T.A.; Ku, X.; Vasudev, V. Co-Pyrolysis of Peanut Shells and Tea Plant Branches: Physicochemical Properties, Synergistic Effect and Thermo-Kinetic Analyses. Bioenergy Res. 2024, 17, 1805–1815. https://doi.org/10.1007/s12155-024-10728-5.
- 113.
Friedman, H.L. Kinetics of thermal degradation of char‐forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. https://doi.org/10.1002/polc.5070060121.
- 114.
Balasundram, V.; Ibrahim, N.; Kasmani, R.M.; et al. Thermogravimetric catalytic pyrolysis and kinetic studies of coconut copra and rice husk for possible maximum production of pyrolysis oil. J. Clean. Prod. 2017, 167, 218–228. https://doi.org/10.1016/j.jclepro.2017.08.173.
- 115.
Fu, W.; Zhang, Y.; Liu, Z.; et al. Kinetic and thermodynamic insights into the catalytic pyrolysis of PP with Fe/Ni catalysts. Energy 2025, 330, 136809. https://doi.org/10.1016/j.energy.2025.136809.
- 116.
Ram, S.; Ku, X.; Vasudev, V. Catalytic pyrolysis of green algae: Influence of catalysts on thermal degradation behavior and product distribution. Biofuels Bioprod. Biorefin. 2025, 19, 1904–1914. https://doi.org/10.1002/bbb.2791.
- 117.
Li, B.Y.; Tee, M.Y.; Nge, K.S.; et al. Comparison Kinetic Analysis between Coats-Redfern and Criado’s Master Plot on Pyrolysis of Horse Manure. Chem. Eng. Trans. 2023, 106, 1273–1278. https://doi.org/10.3303/CET23106213.
- 118.
Gözke, G. Kinetic and thermodynamic analyses based on thermogravimetric pyrolysis of watermelon seed by isoconversional and master plots methods. Renew. Energy 2022, 201, 916–927. https://doi.org/10.1016/j.renene.2022.10.100.
- 119.
Nie, N.; Wang, Y.; Yellezuome, D.; et al. Exploring kinetic and thermodynamic mechanisms of switchgrass pyrolysis using iterative linear integral isoconversional method and master plots approach. Fuel 2023, 338, 127266. https://doi.org/10.1016/j.fuel.2022.127266.
- 120.
Ram, S.; Vasudev, V.; Ku, X. Characterization and kinetic analysis of lignocellulosic and algal biochar combustion. Int. J. Fluid Eng. 2024, 1, 024302. https://doi.org/10.1063/5.0194358.
- 121.
Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional methods as single-step kinetic approximation. Thermochim. Acta 2024, 733, 179692. https://doi.org/10.1016/j.tca.2024.179692.
- 122.
Vyazovkin, S. Misinterpretation of Thermodynamic Parameters Evaluated from Activation Energy and Preexponential Factor Determined in Thermal Analysis Experiments. Thermo 2024, 4, 373–381. https://doi.org/10.3390/thermo4030019.
- 123.
Raza, A.; Khan, W.U.H.; Khoja, A.H.; et al. Thermokinetic investigation of Polyethylene Terephthalate (PET) plastic over biomass fly ash (BFA) catalyst using pyrolysis process through non-isothermal thermogravimetric analysis. Sustain. Chem. Pharm. 2024, 42, 101856. https://doi.org/10.1016/j.scp.2024.101856.
- 124.
Rony, A.H.; Kong, L.; Lu, W.; et al. Kinetics, thermodynamics, and physical characterization of corn stover (Zea mays) for solar biomass pyrolysis potential analysis. Bioresour. Technol. 2019, 284, 466–473. https://doi.org/10.1016/j.biortech.2019.03.049.
- 125.
Gotor, F.J.; Criado, J.M.; Malek, J.; et al. Kinetic analysis of solid-state reactions: The universality of master plots for analyzing isothermal and nonisothermal experiments. J. Phys. Chem. A 2000, 104, 10777–10782. https://doi.org/10.1021/jp0022205.
- 126.
Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; et al. Generalized master plots as a straightforward approach for determining the kinetic model: The case of cellulose pyrolysis. Thermochim. Acta 2013, 552, 54–59. https://doi.org/10.1016/j.tca.2012.11.003.
- 127.
Mehl, M.; Pelucchi, M.; Maffei, L.P.; et al. Developing chemical kinetic models for thermochemical applications. Nat. Protoc. 2025, 135, 11100–11114. https://doi.org/10.1038/s41596-025-01195-z.
- 128.
Polat, S.; Kılıç, Ö.F. Pyrolysis of Chlorella vulgaris: Kinetic analysis, advanced characterization, and bio-oil optimization. J. Environ. Chem. Eng. 2025, 13, 120374. https://doi.org/10.1016/j.jece.2025.120374.
- 129.
Shen, T.; Wu, T.; Liang, Y.; et al. Kinetic analysis and neural network-based prediction of TGA curves during the co-pyrolysis of industrial hemp waste and polyvinyl chloride. J. Anal. Appl. Pyrolysis 2026, 193, 107463. https://doi.org/10.1016/j.jaap.2025.107463.
- 130.
Petrovič, A.; Vohl, S.; Gruber, S.; et al. Thermogravimetric, kinetic and thermodynamic behaviour of raw and hydrothermally pretreated oil cakes during pyrolysis and TG-FTIR analysis of the gaseous products. Renew. Energy 2025, 247, 123041. https://doi.org/10.1016/j.renene.2025.123041.
- 131.
Liu, Y.; Jiang; Huang, Z. Comprehensive pyrolysis study and kinetic analysis of PMMA/H-β zeolite hybrids using the Gaussian deconvolution method. Therm. Sci. Eng. Prog. 2025, 62, 103563. https://doi.org/10.1016/j.tsep.2025.103563.
- 132.
Liu, M.; Tao, J.; Mu, L.; et al. Machine learning-driven predictions of biochar yield and NPK composition: Insights into biomass pyrolysis with data augmentation and model interpretability. Carbon Res. 2025, 4, 62. https://doi.org/10.1007/s44246-025-00229-1.
- 133.
Xiao, K.; Zhu, X. Machine Learning Approach for the Prediction of Biomass Waste Pyrolysis Kinetics from Preliminary Analysis. ACS Omega 2024, 9, 48125–48136. https://doi.org/10.1021/acsomega.4c04649.
- 134.
Zhang, J.; Liu, J.; Evrendilek, F.; et al. TG-FTIR and Py-GC/MS analyses of pyrolysis behaviors and products of cattle manure in CO2 and N2 atmospheres: Kinetic, thermodynamic, and machine-learning models. Energy Convers. Manag. 2019, 195, 346–359. https://doi.org/10.1016/j.enconman.2019.05.019.
- 135.
Potnuri, R.; Suriapparao, D.V.; Rao, C.S.; et al. Effect of dry torrefaction pretreatment of the microwave-assisted catalytic pyrolysis of biomass using the machine learning approach. Renew. Energy 2022, 197, 798–809. https://doi.org/10.1016/j.renene.2022.08.006.
- 136.
Akbari, A.; Sokhansanj, A.; Shfiee, M.; et al. Structural and adsorptive comparison of activated hydrochar and biochar: Machine learning analysis and novel driven kinetic and thermodynamic insight. J. Water Process Eng. 2025, 76, 108164. https://doi.org/10.1016/j.jwpe.2025.108164.
- 137.
Li, J.; Liu, T.; Palansooriya, K.N.; et al. Zeolite-catalytic pyrolysis of waste plastics: Machine learning prediction, interpretation, and optimization. Appl. Energy 2025, 382, 125258. https://doi.org/10.1016/j.apenergy.2024.125258.
- 138.
Mahanta, B.K.; Kumar, S.; Pathak, S.K.; et al. Machine learning-based prediction of high-entropy alloys for hydrogen storage with optimized thermodynamic and kinetic parameters. J. Energy Storage 2025, 139, 118865. https://doi.org/10.1016/j.est.2025.118865.
- 139.
Hazmi, B.; Farooq, H.; Rashid, U.; et al. Characterization and pyrolysis kinetic modelling of lignocellulosic waste from rambutan seeds: A machine learning approach. Biomass Bioenergy 2026, 204, 108426. https://doi.org/10.1016/j.biombioe.2025.108426.
- 140.
Tagade, A.; Kandpal, S.; Singh, S.; et al. Kinetic and thermodynamic analyses of pyrolysis of finger millet (Eleusine coracana) straw through both model-free and model-based methods and application of ANN-based machine learning model to predict thermal degradation. Bioresour. Technol. Rep. 2025, 30, 102139. https://doi.org/10.1016/j.biteb.2025.102139.
- 141.
Wang, S.; Shi, Z.; Jin, Y.; et al. A machine learning model to predict the pyrolytic kinetics of different types of feedstocks. Energy Convers. Manag. 2022, 260, 115613. https://doi.org/10.1016/j.enconman.2022.115613.
- 142.
Khandelwal, K.; Nanda, S.; Dalai, A.K. Machine learning to predict the production of bio-oil, biogas, and biochar by pyrolysis of biomass: A review. Environ. Chem. Lett. 2024, 22, 2669–2698. https://doi.org/10.1007/s10311-024-01767-7.
- 143.
Cardarelli, A.; Ciambella, M.; Fornai, P.; et al. Kinetic analysis and prediction modeling by advanced machine learning of pyrolysis of dairy cattle manure from conventional and organic systems. Biomass Bioenergy 2025, 202, 108247. https://doi.org/10.1016/j.biombioe.2025.108247.
- 144.
Tiwari, A.; Rao, C.S.; Jammula, K.; et al. Kinetic analysis and machine learning insights in the production of biochar from Artocarpus heterophyllus (jackfruit) through pyrolysis. Biomass Bioenergy 2025, 201, 108125. https://doi.org/10.1016/j.biombioe.2025.108125.
- 145.
Zhong, Y.; Liu, F.; Huang, G.; et al. Thermogravimetric experiments based prediction of biomass pyrolysis behavior: A comparison of typical machine learning regression models in Scikit-learn. Mar. Pollut. Bull. 2024, 202, 116361. https://doi.org/10.1016/j.marpolbul.2024.116361.
- 146.
Gao, W.; Zhang, Y.; Wang, Y.; et al. Pyrolysis characteristics and kinetic analysis of coating pitch derived from ethylene tar using model-free and model-fitting methods. J. Anal. Appl. Pyrolysis 2024, 183, 106803. https://doi.org/10.1016/j.jaap.2024.106803.
- 147.
Bharti, B.; Kandpal, S.; Sawarkar, A.N.; et al. Holistic approach for sequential transesterification and pyrolysis of microalgal biomass: Kinetic and thermodynamic analysis of pyrolysis using model-free and model-based approaches. Renew. Energy 2024, 235, 121319. https://doi.org/10.1016/j.renene.2024.121319.
- 148.
Sbirrazzuoli, N. Kinetic analysis of complex chemical reactions by coupling model-free and model-fitting analysis. Thermochim. Acta 2023, 719, 179416. https://doi.org/10.1016/j.tca.2022.179416.
- 149.
Budrugeac, P. On the use of the model-free way method for kinetic analysis of thermoanalytical data—Advantages and limitations. Thermochim. Acta 2021, 706, 179063. https://doi.org/10.1016/j.tca.2021.179063.
- 150.
Aggarwal, N.; Pham, H.L.; Ranjan, B.; et al. Microbial engineering strategies to utilize waste feedstock for sustainable bioproduction. Nat. Rev. Bioeng. 2024, 2, 155–174. https://doi.org/10.1038/s44222-023-00129-2.
- 151.
Mastry, M.C.; Dorazio, L.; Fu, J.C.; et al. Processing renewable and waste-based feedstocks with fluid catalytic cracking: Impact on catalytic performance and considerations for improved catalyst design. Front. Chem. 2023, 11, 1067488. https://doi.org/10.3389/fchem.2023.1067488.
- 152.
Ullah, K.; Sharma, V.K.; Ahmad, M.; et al. The insight views of advanced technologies and its application in bio-origin fuel synthesis from lignocellulose biomasses waste, a review. Renew. Sustain. Energy Rev. 2018, 82, 3992–4008. https://doi.org/10.1016/j.rser.2017.10.074.
- 153.
Kumar, R.; Strezov, V.; Weldekidan, H.; et al. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. https://doi.org/10.1016/j.rser.2020.109763.
- 154.
Escalante, J.; Chen, W.H.; Tabatabaei, M.; et al. Pyrolysis of lignocellulosic, algal, plastic, and other biomass wastes for biofuel production and circular bioeconomy: A review of thermogravimetric analysis (TGA) approach. Renew. Sustain. Energy Rev. 2022, 169, 112914. https://doi.org/10.1016/j.rser.2022.112914.
- 155.
Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. https://doi.org/10.1016/j.fuel.2016.04.106.
- 156.
Kumar, M.; Sun, Y.; Rathour, R.; et al. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. https://doi.org/10.1016/j.scitotenv.2020.137116.
- 157.
Rebrov, E.; Panjabi, R.; Mong, G.R.; et al. Reaction kinetics and product distributions in thermal and catalytic pyrolysis of agricultural mulch films over HZSM-5 zeolite. Chem. Eng. J. 2025, 522, 167458. https://doi.org/10.1016/j.cej.2025.167458.
- 158.
Wang, S.; Lin, H.; Ru, B.; et al. Kinetic modeling of biomass components pyrolysis using a sequential and coupling method. Fuel 2016, 185, 763–771. https://doi.org/10.1016/j.fuel.2016.08.037.
- 159.
Wang, C.; Li, L.; Zeng, Z.; et al. Catalytic performance of potassium in lignocellulosic biomass pyrolysis based on an optimized three-parallel distributed activation energy model. Bioresour. Technol. 2019, 281, 412–420. https://doi.org/10.1016/j.biortech.2019.02.118.
- 160.
Kavade, O.G.; Dhepe, P.L.; Devi, N.R.; et al. Experimental investigation and lumped kinetic modeling studies for upcycling of polyolefins. J. Indian Chem. Soc. 2025, 102, 102127. https://doi.org/10.1016/j.jics.2025.102127.
- 161.
Suleiman, M.Y.; Benedetto, E.; Piazza, V.; et al. Kinetic modelling of biomass pyrolysis: A new lumped scheme for xylan-based hardwood hemicellulose. Energy Convers. Manag. X 2025, 27, 101130. https://doi.org/10.1016/j.ecmx.2025.101130.
- 162.
Tanha, M.A.; Kazemeini, M.; Hosseinpour, V.; et al. A genetic algorithm aimed at developing a lumped kinetic model for conversion of ethyl mercaptan into hydrogen sulfide on an H-ZSM-5 catalyst. Results Eng. 2025, 27, 105784. https://doi.org/10.1016/j.rineng.2025.105784.
- 163.
Bach, Q.V.; Chen, W.H. Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): A state-of-the-art review. Bioresour. Technol. 2017, 246, 88–100. https://doi.org/10.1016/j.biortech.2017.06.087.
- 164.
Wang, W.; Li, Y.; Li, Y.; et al. Activation energy calculation method for waste plastic pyrolysis based on a difference-weighted model. Fuel 2026, 405, 136754. https://doi.org/10.1016/j.fuel.2025.136754.
- 165.
He, X.C.; Chen, D.Z. ReaxFF MD study on the early stage co-pyrolysis of mixed PE/PP/PS plastic waste. J. Fuel Chem. Technol. 2022, 50, 346–356. https://doi.org/10.1016/S1872-5813(21)60161-5.
- 166.
Handawy, M.K.M.; Abdellatief, T.M.M.; Duan, X.; et al. A hybrid AI-kinetic framework for predicting the pyrolysis of food packaging plastic waste: Integrating TGA, model-free kinetics, and artificial neural networks. Appl. Energy Combust. Sci. 2025, 24, 100418. https://doi.org/10.1016/j.jaecs.2025.100418.
- 167.
Riaz, S.; Ahmad, N.; Farooq, W; et al. Catalytic pyrolysis of HDPE for enhanced hydrocarbon yield: A boosted regression tree assisted kinetics study for effective recycling of waste plastic. Digit. Chem. Eng. 2025, 14, 100213. https://doi.org/10.1016/j.dche.2024.100213.
- 168.
Martínez-Narro, G.; Royston, N.J.; Billsborough, K.L.; et al. Kinetic modelling of mixed plastic waste pyrolysis. Chem. Thermodyn. Therm. Anal. 2023, 9, 100105. https://doi.org/10.1016/j.ctta.2023.100105.
- 169.
Armenise, S.; Luing, W.S.; Vázquez, M.A.R.; et al. Tailoring catalyst acidity and hierarchical pore structure for enhanced BTX yields in plastic waste pyrolysis. J. Environ. Chem. Eng. 2025, 13, 119493. https://doi.org/10.1016/j.jece.2025.119493.
- 170.
Das, P. Pyrolysis study of a waste plastic mixture through different kinetic models using isothermal and nonisothermal mechanism. RSC Adv. 2024, 14, 25599–25618. https://doi.org/10.1039/d4ra04957h.
- 171.
Pyo, S.; Kim, Y.M.; Park, Y.; et al. Catalytic pyrolysis of polypropylene over Ga loaded HZSM-5. J. Ind. Eng. Chem. 2021, 103, 136–141. https://doi.org/10.1016/j.jiec.2021.07.027.
- 172.
Majidian, N.; Saleh, M.; Samipourgiri, M. Kinetics study of catalytic pyrolysis of polystyrene polymer using response surface method. Iran. Polym. J. 2024, 33, 1793–1806. https://doi.org/10.1007/s13726-024-01362-1.
- 173.
Li, M.; Jia, Y.; Chen, D.; et al. Exploring pyrolysis mechanism of waste PET in different degrees of polymerization to regulate the pyrolysis products. Polym. Degrad. Stab. 2025, 233, 111175. https://doi.org/10.1016/j.polymdegradstab.2025.111175.
- 174.
Miskolczi, N.; Gao, N.B.; Quan, C. Transformation of biomass and waste plastic mixtures into hydrocarbon oils and gases by pyrolysis using different reactor temperatures and pressures. J. Anal. Appl. Pyrolysis 2024, 180, 106520. https://doi.org/10.1016/j.jaap.2024.106520.
- 175.
Chen, G.; Cao, X.; Che, Y.; et al. Synergistic effect on low-pressure pyrolysis of biomass-plastic mixture as representative of Tibetan tourism solid waste. J. Anal. Appl. Pyrolysis 2023, 175, 106181. https://doi.org/10.1016/j.jaap.2023.106181.
- 176.
Wu, Z.; Li, Y.; Zhang, B.; et al. Co-pyrolysis behavior of microalgae biomass and low-rank coal: Kinetic analysis of the main volatile products. Bioresour. Technol. 2019, 271, 202–209. https://doi.org/10.1016/j.biortech.2018.09.076.
- 177.
Cui, Q.; Ma, X.; Nakano, K.; et al. Effect of blending on hydrotreating reactivities of atmospheric residues: Synergistic effects. Fuel 2021, 293, 120429. https://doi.org/10.1016/j.fuel.2021.120429.
- 178.
Vo, T.A.; Tran, Q.K.; Ly, H.V.; et al. Co-pyrolysis of lignocellulosic biomass and plastics: A comprehensive study on pyrolysis kinetics and characteristics. J. Anal. Appl. Pyrolysis 2022, 163, 105464. https://doi.org/10.1016/j.jaap.2022.105464.
- 179.
Xu, G.; Yang, X.; Zhang, J.; et al. Nitrogen-sulfur transport, products, and synergistic effects in the co-pyrolysis of the CaO/K2FeO4 conditioned sludge and chlorella. J. Energy Inst. 2025, 125, 102421. https://doi.org/10.1016/j.joei.2025.102421.
- 180.
Li, Y.; Guo, Z.; Zhu, H.; et al. Thermal behavior and K–Fe synergistic effects in the catalytic pyrolysis of Chinese herb residues for H2-rich syngas. Int. J. Hydrogen Energy 2025, 196, 152543. https://doi.org/10.1016/j.ijhydene.2025.152543.
- 181.
Xu, S.; Cao, B.; Uzoejinwa, B.B.; et al. Synergistic effects of catalytic co-pyrolysis of macroalgae with waste plastics. Process Saf. Environ. Prot. 2020, 137, 34–48. https://doi.org/10.1016/j.psep.2020.02.001.
- 182.
Dussan, K.; Dooley, S.; Monaghan, R.F.D.; et al. New Pseudo-Components of Hemicellulose and Lignin. In Proceedings of the 25th European Biomass Conference and Exhibition, Stockholm, Sweden 12–15 June2017; pp. 971–979. https://doi.org/10.5071/25thEUBCE2017-3BO.11.3.
- 183.
Sipra, A.T.; Gao, N.; Sarwar, H. Municipal solid waste (MSW) pyrolysis for bio-fuel production: A review of effects of MSW components and catalysts. Fuel Process. Technol. 2018, 175, 131–147. https://doi.org/10.1016/j.fuproc.2018.02.012.
- 184.
Sawatmongkhon, B.; Promhuad, P.; Wongkhorsub, C.; et al. Kinetic analysis of diesel particulate matter oxidation using a multi-step reaction model, components peak deconvolution, and isoconversional methods. Fuel 2025, 390, 134644. https://doi.org/10.1016/j.fuel.2025.134644.
- 185.
Kumar, A. Co-pyrolysis of microalgae residue and sewage sludge: An in-depth characterization of kinetics, drivers, and gas-oil-char behaviors. J. Anal. Appl. Pyrolysis 2024, 179, 106438. https://doi.org/10.1016/j.jaap.2024.106438.
- 186.
Adhikari, R.; Rabinovitch, J.; Parziale, N.; et al. Hierarchical kinetic analysis for development of a reduced-order model of the multi-step thermal decomposition of munitions wastewater. Thermochim. Acta 2025, 750, 180038. https://doi.org/10.1016/j.tca.2025.180038.
- 187.
Locaspi, A.; Faravelli, T. Generalized derivation of multi-step kinetic models for polymer condensed-phase pyrolysis: Application to poly(ethylene terephthalate). Proc. Combust. Inst. 2025, 41, 105978. https://doi.org/10.1016/j.proci.2025.105978.
- 188.
Liu, C.; Zhang, B.; Bian, Y.; et al. Synergistic effect and kinetic analysis of catalytic co-pyrolysis of waste cotton swabs and non-woven masks. J. Anal. Appl. Pyrolysis 2022, 167, 105677. https://doi.org/10.1016/j.jaap.2022.105677.
- 189.
Wang, S.; Wu, K.; Yu, J.; et al. Kinetic and thermodynamic analysis of biomass catalytic pyrolysis with nascent biochar in a two-stage reactor. Combust. Flame 2023, 251, 112671. https://doi.org/10.1016/j.combustflame.2023.112671.
- 190.
Zheng, Y.; Li, D.; Pei, T.; et al. Mechanism of synergistic effects and kinetic analysis in bamboo-LDPE waste ex-situ catalytic co-pyrolysis for enhanced aromatic hydrocarbon production via CeZrAl and HZSM-5 dual catalyst. J. Environ. Chem. Eng. 2022, 10, 107479. https://doi.org/10.1016/j.jece.2022.107479.
- 191.
Okonsky, S.T.; Krishna, J.V.J.; Lee, D.H.; et al. Kinetic modelling and measurement of catalyst deactivation for the catalytic co-pyrolysis of PP and PET with HZSM-5. Appl. Catal. A Gen. 2025, 708, 120574. https://doi.org/10.1016/j.apcata.2025.120574.
- 192.
Huang, S.; Su, Y.; Luo, W.; et al. Kinetic analysis and in-situ no support catalytic pyrolysis product distribution of Chinese herb residue. J. Anal. Appl. Pyrolysis 2021, 156, 105114. https://doi.org/10.1016/j.jaap.2021.105114.
- 193.
Fong, M.J.B.; Loy, A.C.M.; Chin, B.L.F.; et al. Catalytic pyrolysis of Chlorella vulgaris: Kinetic and thermodynamic analysis. Bioresour. Technol. 2019, 289, 121689. https://doi.org/10.1016/j.biortech.2019.121689.
- 194.
Panicker, T.F.; Gupta, R.; Mishra, R.K.; et al. Thermo-catalytic pyrolysis and kinetic study of non-edible castor seeds into renewable liquid fuel and value-added chemicals. Energy Rep. 2025, 14, 2280–2295. https://doi.org/10.1016/j.egyr.2025.08.050.
- 195.
Yang, J.; Xu, X.; Liang, S.; et al. Enhanced hydrogen production in catalytic pyrolysis of sewage sludge by red mud: Thermogravimetric kinetic analysis and pyrolysis characteristics. Int. J. Hydrogen Energy 2018, 43, 7795–7807. https://doi.org/10.1016/j.ijhydene.2018.03.018.
- 196.
Dwivedi, P.; Rathore, A.K. Thermogravimetric kinetic study of catalytic and non-catalytic pyrolysis of PET bottles and micro carbon rod formation. J. Chem. Thermodyn. 2025, 209, 107530. https://doi.org/10.1016/j.jct.2025.107530.
- 197.
Sharma, H.; Chakinala, N.; Thota, C.; et al. Exploring the catalytic conversion of pyrolytic wax residue: Kinetics and co-pyrolysis. J. Anal. Appl. Pyrolysis 2026, 193, 107331. https://doi.org/10.1016/j.jaap.2025.107331.
- 198.
Kumar, R.; Manoth, A.; Mondal, M.K. Pyrolysis behavior of native and Ni-Fe treated biomass: Kinetic modeling and thermodynamic insights. Therm. Sci. Eng. Prog. 2025, 66, 104026. https://doi.org/10.1016/j.tsep.2025.104026.
- 199.
Farooq, W.; Ali, I.; Naqvi, S.R.; et al. Evolved Gas Analysis and Kinetics of Catalytic and Non-Catalytic Pyrolysis of Microalgae Chlorella sp. Biomass with Ni/θ-Al2O3 Catalyst via Thermogravimetric Analysis. Front. Energy Res. 2021, 9, 775037. https://doi.org/10.3389/fenrg.2021.775037.
- 200.
Eimontas, J.; Yousef, S.; Striūgas, N.; et al. Catalytic pyrolysis kinetic behaviour and TG-FTIR-GC–MS analysis of waste fishing nets over ZSM-5 zeolite catalyst for caprolactam recovery. Renew. Energy 2021, 179, 1385–1403. https://doi.org/10.1016/j.renene.2021.07.143.
- 201.
Wang, P.; Shen, Y. Catalytic pyrolysis of cellulose and chitin with calcined dolomite—Pyrolysis kinetics and products analysis. Fuel 2022, 312, 122875. https://doi.org/10.1016/j.fuel.2021.122875.
- 202.
Yu, L.; Shi, X.; Zhang, S.; et al. A comprehensive investigation of kinetic and thermodynamic analysis to catalytic pyrolysis bamboo with N-doped biochar. Fuel 2025, 396, 135312. https://doi.org/10.1016/j.fuel.2025.135312.
- 203.
Oliveira, C.C.; dos Santos, G.E.S.; Vieira, L.G.M.; et al. Catalytic Co-pyrolysis of soybean husk and high-density polyethylene: Artificial neural network modeling and synergistic approach for enhanced gasoline-range biofuel production using zeolite HZSM-5. Renew. Energy 2026, 256, 124148. https://doi.org/10.1016/j.renene.2025.124148.
- 204.
Hassan, H.; Hameed, B.H.; Lim, J.K. Co-pyrolysis of sugarcane bagasse and waste high-density polyethylene: Synergistic effect and product distributions. Energy 2020, 191, 116545. https://doi.org/10.1016/j.energy.2019.116545.
- 205.
Nardella, F.; Bellavia, S.; Mattonai, M.; et al. Co-pyrolysis of biomass and plastic: Synergistic effects and estimation of elemental composition of pyrolysis oil by analytical pyrolysis–gas chromatography/mass spectrometry. Bioresour. Technol. 2022, 354, 127170. https://doi.org/10.1016/j.biortech.2022.127170.
- 206.
Özsin, G.; Pütün, A.E. TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers. Manag. 2019, 182, 143–153. https://doi.org/10.1016/j.enconman.2018.12.060.
- 207.
Nawaz, A.; Razzak, S.A. Synergism, pyrolysis performance, product distribution and characteristics in the co-pyrolysis of date palm waste and polyethylene foam: Harnessing the potential of plastics and biomass valorization. Carbon Resour. Convers. 2025, 8, 100312. https://doi.org/10.1016/j.crcon.2025.100312.
- 208.
Yang, C.; Li, C.; Liu, H.; et al. Co-pyrolysis of waste office paper and high-density polyethylene: Product distribution, kinetics and reaction mechanism. J. Energy Inst. 2025, 120, 102105. https://doi.org/10.1016/j.joei.2025.102105.
- 209.
Fu, J.; Wu, X.; Liu, J.; et al. Co-circularity of spent coffee grounds and polyethylene via co-pyrolysis: Characteristics, kinetics, and products. Fuel 2023, 337, 127061. https://doi.org/10.1016/j.fuel.2022.127061.
- 210.
Jae, J.; Tompsett, G.A.; Foster, A.J.; et al. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. https://doi.org/10.1016/j.jcat.2011.01.019.
- 211.
French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. https://doi.org/10.1016/j.fuproc.2009.08.011.
- 212.
Carlson, T.R.; Cheng, Y.T.; Jae, J.; et al. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2011, 4, 145–161. https://doi.org/10.1039/c0ee00341g.
- 213.
Ochoa, A.; Bilbao, J.; Gayubo, A.G.; et al. Coke formation and deactivation during catalytic reforming of biomass and waste pyrolysis products: A review. Renew. Sustain. Energy Rev. 2020, 119, 109600. https://doi.org/10.1016/j.rser.2019.109600.
- 214.
Ranzi, E.; Debiagi, P.E.A.; Frassoldati, A. Mathematical Modeling of Fast Biomass Pyrolysis and Bio-Oil Formation. Note II: Secondary Gas-Phase Reactions and Bio-Oil Formation. ACS Sustainable Chem. Eng. 2017, 5, 2882–2896. https://doi.org/10.1021/acssuschemeng.6b03098.
- 215.
Kanchan, D.R.; Banerjee, A. Role of support in phenol hydrodeoxygenation over supported Pd catalysts: Insights from first-principles based microkinetic modelling. J. Catal. 2025, 450, 116266. https://doi.org/10.1016/j.jcat.2025.116266.
- 216.
Gracia, A.; Lozano-Reis, P.; Huarte-Larrañaga, F.; et al. CO2 hydrogenation on Ni(111): Microkinetic modelling vs. kinetic Monte Carlo simulations—Choosing the right approach for unravelling reaction kinetics. RSC Sustain. 2025, 3, 3499–3512. https://doi.org/10.1039/d5su00240k.
- 217.
Liu, D.; Bai, Y.; Chen, D.; et al. Catalytic pyrolysis of n-paraffin coexisting with olefin over ZSM-5 based catalysts: Experiments, kinetics, and DFT calculations. Chem. Eng. Sci. 2026, 320, 122533. https://doi.org/10.1016/j.ces.2025.122533.
- 218.
Chen, D.; Liu, D.; He, H.; et al. Rational tuning of monomolecular, bimolecular and aromatization pathways in the catalytic pyrolysis of hexane on ZSM-5 from a first-principles-based microkinetics analysis. Fuel 2024, 366, 131368. https://doi.org/10.1016/j.fuel.2024.131368.
- 219.
Chen, D.; Wang, H.; Wei, J.; et al. Reaction mechanism and microkinetics of 1-hexene catalytic pyrolysis on HZSM-5: A first-principles study. Chem. Eng. Sci. 2023, 282, 119220. https://doi.org/10.1016/j.ces.2023.119220.
- 220.
Ugwu, L.I.; Morgan, Y.; Ibrahim, H. Application of density functional theory and machine learning in heterogenous-based catalytic reactions for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 2245–2267. https://doi.org/10.1016/j.ijhydene.2021.10.208.
- 221.
Wang, Y.; Yao, Z.; Deng, S.; et al. Unravelling the reaction mechanism for the AP decomposition over MgO: A density functional theory study and microkinetic simulation. Mater. Today Commun. 2024, 39, 108658. https://doi.org/10.1016/j.mtcomm.2024.108658.
- 222.
Luo, H.; Wang, X.; Krochmalny, K.; et al. Assessments and analysis of lumped and detailed pyrolysis kinetics for biomass torrefaction with particle-scale modeling. Biomass Bioenergy 2022, 166, 106619. https://doi.org/10.1016/j.biombioe.2022.106619.
- 223.
Rodríguez, S.; Trueba, D.; Escribano, M.; et al. Six-lump kinetic model for plastic pyrolysis oil (PPO) and vacuum gasoil (VGO) blend hydroprocessing considering selective catalyst deactivation. Catal. Today 2025, 457, 115341. https://doi.org/10.1016/j.cattod.2025.115341.
- 224.
Bjelić, A.; Garbarino, L.I.; Grilc, M.; et al. Continuous lumping modelling of reductive lignin depolymerization kinetics in the presence of a Ru/C catalyst. Chem. Eng. Sci. 2025, 317, 122096. https://doi.org/10.1016/j.ces.2025.122096.
- 225.
Vicente, H.; Aguayo, A.T.; Castaño, P.; et al. Dual-cycle-based lumped kinetic model for methanol-to-aromatics (MTA) reaction over H-ZSM-5 zeolites of different Si/Al ratio. Fuel 2024, 361, 130704. https://doi.org/10.1016/j.fuel.2023.130704.
- 226.
Jaroenkhasemmeesuk, C.; Tippayawong, N.; Shimpalee, S.; et al. Improved simulation of lignocellulosic biomass pyrolysis plant using chemical kinetics in Aspen Plus® and comparison with experiments. Alexandria Eng. J. 2023, 63, 199–209. https://doi.org/10.1016/j.aej.2022.07.060.
- 227.
Khongprom, P.; Ratchasombat, S.; Wanchan, W.; et al. Scaling of catalytic cracking fluidized bed downer reactor based on CFD simulations—Part II: Effect of reactor scale. RSC Adv. 2022, 12, 21394–21405. https://doi.org/10.1039/d2ra03448d.
- 228.
Ciesielski, P.N.; Pecha, M.B.; Thornburg, N.E.; et al. Bridging Scales in Bioenergy and Catalysis: A Review of Mesoscale Modeling Applications, Methods, and Future Directions. Energy Fuels 2021, 35, 14382–14400. https://doi.org/10.1021/acs.energyfuels.1c02163.
- 229.
Mutlu, Ö.Ç.; Zeng, T. Challenges and Opportunities of Modeling Biomass Gasification in Aspen Plus: A Review. Chem. Eng. Technol. 2020, 43, 1674–1689. https://doi.org/10.1002/ceat.202000068.
- 230.
Micale, D.; Uglietti, R.; Bracconi, M.; et al. Coupling euler-euler and microkinetic modeling for the simulation of fluidized bed reactors: An application to the oxidative coupling of methane. Ind. Eng. Chem. Res. 2021, 60, 6687–6697. https://doi.org/10.1021/acs.iecr.0c05845.
- 231.
Nestler, F.; Müller, V.P.; Ouda, M.; et al. A novel approach for kinetic measurements in exothermic fixed bed reactors: Advancements in non-isothermal bed conditions demonstrated for methanol synthesis. React. Chem. Eng. 2021, 6, 1092–1107. https://doi.org/10.1039/d1re00071c.
- 232.
Cheah, Y.W.; Salam, M.A.; Sebastian, J.; et al. Upgrading of triglycerides, pyrolysis oil, and lignin over metal sulfide catalysts: A review on the reaction mechanism, kinetics, and catalyst deactivation. J. Environ. Chem. Eng. 2023, 11, 109614. https://doi.org/10.1016/j.jece.2023.109614.
- 233.
Jiang, W.; Ma, X.; Zhang, D.; et al. Highly efficient catalysts for hydrogen generation through methanol steam reforming: A critical analysis of modification strategies, deactivation, mechanisms and kinetics. J. Ind. Eng. Chem. 2024, 130, 54–72. https://doi.org/10.1016/j.jiec.2023.09.043.
- 234.
Vares, M.; Sari, A.; Yaripour, F. Intrinsic reaction and deactivation kinetics of Methanol-to-Propylene process (MTP) over an industrial ZSM-5 catalyst. Fuel 2025, 383, 133861. https://doi.org/10.1016/j.fuel.2024.133861.
- 235.
Muhammad, I.; Makwashi, N.; Ahmed, T.G.; et al. A Mechanistic Model on Catalyst Deactivation by Coke Formation in a CSTR Reactor. Processes 2023, 11, 944. https://doi.org/10.3390/pr11030944.
- 236.
Noor, E.; Flamholz, A.; Liebermeister, W.; et al. A note on the kinetics of enzyme action: A decomposition that highlights thermodynamic effects. FEBS Lett. 2013, 587, 2772–2777. https://doi.org/10.1016/j.febslet.2013.07.028.
- 237.
Chaouati, N.; Soualah, A.; Pinard, L. Coke toxicities, descriptors of the deactivating effect of coke on microporous and desilicated mordenite zeolite. React. Kinet. Mech. Catal. 2023, 136, 2243–2257. https://doi.org/10.1007/s11144-023-02447-3.
- 238.
Langer, M.; Waldner, M.; Freund, H. Modeling of the combined deactivation and reaction kinetic behavior of an impregnated Ni/Al2O3 catalyst in the CO2 methanation reaction. Chem. Eng. J. 2025, 524, 169363. https://doi.org/10.1016/j.cej.2025.169363.
- 239.
Wei, H.L.; Jihou, Z.Q.; Liu, X.Q.; et al. Long-term alkaline deactivation of Cu-based catalyst in formaldehyde ethynylation: Mechanism and kinetic analysis. Chem. Eng. J. 2025, 525, 170651. https://doi.org/10.1016/j.cej.2025.170651.
- 240.
Deng, Z.H.; Shi, R.; Ren, L.; et al. Catalyst deactivation model involving autocatalytic effect for the residue hydrotreating process. Pet. Sci. 2025, 22, 3447–3460. https://doi.org/10.1016/j.petsci.2025.04.031.
- 241.
Khatri, I.; Garg, A. Use of heterogeneous activated carbon supported copper catalyst for catalytic wet oxidation of biomethanated spent wash: Reaction kinetics, catalyst stability, catalyst deactivation kinetics and biochemical methane potential. J. Water Process Eng. 2021, 44, 102387. https://doi.org/10.1016/j.jwpe.2021.102387.
- 242.
Nicola, B.P.; Giner, E.A.; Romay, M.; et al. Challenging the deactivation resistance of Co/Cu-dendritic ZSM-5 zeolites in methane pyrolysis for clean hydrogen. Microporous Mesoporous Mater. 2025, 401, 113939. https://doi.org/10.1016/j.micromeso.2025.113939.
- 243.
Gromotka, Z.; Yablonsky, G.; Ostrovskii, N.; et al. Integral Characteristic of Complex Catalytic Reaction Accompanied by Deactivation. Catalysts 2022, 12, 1283. https://doi.org/10.3390/catal12101283.

This work is licensed under a Creative Commons Attribution 4.0 International License.


