Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
A Review of Renewable Energy and Storage Technologies for Automotive Applications
Xiangnan Yu 1, Yuhai Jin 1, Heli Liu 1, Arnav Rai 1, Michelle Kostin 1, Dimitrios Chantzis 1, Denis J. Politis 2, and Liliang Wang 1,*
1 Department of Mechanical Engineering, Imperial College London, London SW7 2AZ, UK
2 Department of Mechanical and Manufacturing Engineering, University of Cyprus, Nicosia, 1678, Cyprus
* Correspondence: liliang.wang@imperial.ac.uk
Received: 29 September 2022
Accepted: 9 November 2022
Published: 25 December 2022
Abstract: Transportation, as the backbone of the modern economy, continues to be a significant contributing factor to environmental pollution and poor air quality. Whilst numerous efforts have been made to address energy consumption and emissions through the manufacturing and end use stages, the use of fossil fuels continues to grow at an exponential rate. A key target area to assist with fuel consumption reduction targets is the implementation of renewable energy combined with energy storage technologies. The aim of this review is to investigate various means of production for renewable energy and energy storage technologies with the specific focus on the automotive industry.
Keywords:
renewable energy battery electric vehicle (EV) embodied energy storage structural battery automotive industry1. Introduction
The issue of climate change corresponding with rising greenhouse gas emissions is one of the most significant challenges faced by the world today. Global warming caused by human-related emissions has already brought in catastrophic consequences through rising sea levels, extreme weather events, and more frequent wildfires and floods. The automotive industry poses an enormous challenge for decarbonisation and currently accounts for 21% of total global greenhouse gas emissions [1]. Without active intervention, this number is set to increase further. With rising income levels and population growth, the global transport sector is expected to double by 2070. Reducing greenhouse gas emissions to prevent further climate instability requires a rapid transition away from carbon-based fuels. Therefore, there is an incentive to explore renewable energy and energy storage technologies for automotive applications which can mitigate the rise in future greenhouse gas emissions.
As part of the present review, the renewable energy generation methods included were solar, wind, and hydroelectric power. These low-carbon resources were deemed as the most influential based on their significant contribution to the global energy mix [2] and their greater annual power capacity compared to other renewables [3].
Although energy generation is essential, the ability to store this energy is critical to the success of a renewable economy. At present, efficient energy storage presents numerous challenges and effective energy storage solutions are critical to further penetration of renewable assets such as solar and wind [4]. It is anticipated that increasing flexibility of supply through storage will increase utilisation of renewable power. This would facilitate a cost-effective transition away from fossil fuels by automotive applications. In the present study, centralized energy storage technologies–specifically lithium-ion batteries (LIBs) are discussed since they are the most widely used battery storage technology found in electric vehicles (EVs). Moreover, the demand for LIBs is expected to increase exponentially as the EV market expands, with projections suggesting a rise from 580 GWh in 2021 up to 9300 GWh in 2030 [5]. As an ideal alternative, distributed energy storage realized by embodied energy storage technology is also discussed. Although distributed energy storage technologies remain mostly in the research phase, they offer great potential to enable volume and mass savings, thus enhancing the efficiency and range of EVs in the future.
2. Overview of Renewable Energy
To address the impact of climate change, there has been substantial development and investment of technologies aimed at clean and renewable energy, including solar photovoltaic, geothermal, solar thermal, hydroelectric, biofuels and ocean power.
Among renewable technologies, hydropower accounts for the largest renewable energy source of electricity, generating more than all other renewable technologies combined [6]. Solar and wind sources have also been major contenders in the market due to commercial acceptance and technological maturity. As shown in Figure 1, the percentage of renewable energy in global electricity generation is growing from 20.3% in 2011 to 28.5% in 2021 [7,8]. Hydroelectric, wind and solar energy are three major sources for renewable electricity generation.

Figure 1. Renewable Energy Share of Global Electricity Production (2011, 2016, 2021).
Besides, the capacity of these sources is growing at an ever-increasing rate as shown in Figure 2. In 2020, the total addition of renewable energy capacity was more than 256 GW, including 139 GW of solar power, 93 GW of wind power, and 20 GW of hydropower [3].

Figure 2. Annual Capacity Additions of Renewable Energy Sources. Reprinted with permission from [3].
2.1. Hydroelectric Energy
Hydroelectric generation is one of the most widely used and reliable renewable energy sources. It is generated by transferring water between two reservoirs at different heights through a turbine (Figure 3). The fast-flowing or falling water drives turbines at the end of its passage which is coupled to the rotor of an electrical generator [9]. A set of three-phase voltages are then induced in the stator of the electrical generator. Step up transformers are then used to increase the alternating voltage, making it more suitable for long-distance transmission.

Figure 3. Hydropower Energy Generation System. Reprinted with permission from [10].
In 2020, the total installed capacity of hydroelectric power reached its greatest level, at an overall capacity of 4425.43 TWh [7,8], which is approximately 60% of all renewable electricity generated [11]. As shown in Figure 4 [7,8], from 2016 to 2021, hydroelectric power remained the largest renewable and still exceeding the energy from all other renewable electricity combined. However, solar and wind energy capacity has grown at a tremendous rate, where the percentage of hydropower in renewable electricity generation falls from 68.29% in 2016 to 53.74% in 2021.

Figure 4. Hydropower Amount and Its Percentage of Renewable Electricity from 2016 to 2021.
As also shown in Figure 5, the additional annual installed capacity for hydropower is considerably smaller than non-hydro renewables, specifically solar and wind energy. However, hydropower is still an important and vast renewable source. In 2020, electricity generated by using renewable energy was approximately 28% of the total electricity production, with hydropower being 16.8% of the world’s total electricity generation.

Figure 5. Energy Mix for Electricity Production. Reprinted with permission from [3].
Unlike solar and wind energy, hydropower is not an intermittent resource and provides a constant power output. This leads to several advantages including the ability to provide ancillary services to the grid such as frequency regulation, which is vital for balancing energy supply and demand [12]. This will become increasingly important when managing future power networks with greater flexibility and more distributed electricity generation.
Many large-scale hydroelectric plants are currently in operation, such as the Three Gorges dam in China with a capacity of 22.5 GW, and Itaipu on the border between Brazil and Paraguay with a capacity of 14 GW. Possible reasons for the slower annual capacity addition of hydroelectric power compared to wind and solar could include its large capital cost and long lead times for construction [13]. Furthermore, construction of large-scale dams can have huge environmental and social impacts such as destroying wildlife habitats and displacing communities [14]. Creating artificial reservoirs traps vegetation under water, which decays and releases methane into the atmosphere. This counteracts the reduction in emissions associated with renewable resources since methane is a much more potent greenhouse gas than carbon dioxide, with a global warming potential 25 times larger [15].
Therefore, the future outlook of hydroelectric power is likely to include smaller scale ‘micro-hydropower’ plants as discussed by Somani et al. [16], which has smaller environmental impact. Furthermore, pumped-hydro storage is a promising option to exploit the high conversion efficiency of hydroelectric power while minimising environmental impact. These plants make use of excess power generated during periods of low demand, to pump water to an upper reservoir, and such power can then be released to generate extra renewable electricity during high demand periods. With a very high round-trip efficiency of 70-80%, such electricity provides an effective form of energy storage, which could be used to provide regulation services to the grid [13].
2.2. Wind Energy
In the last decade, the cost of producing wind energy has dropped by 56% [17]. In 2021, the current total capacity of wind energy has reached 743 GW globally, helping to reduce over 1.1 billion tonnes of CO2 generation annually [18]. The growth is also reflected in the annual wind energy generation in 2020 of 1591 TWh —— an increase of 360% compared to 2010 levels [19]. Projections indicate that this growth will continue, with 116 GW of installed capacity to be added to the existing 236 GW from 2022-2026 in Europe [20]. The incentive to pursue this option is demonstrated by the fact that 93 GW of installed capacity was implemented globally in 2020 despite the COVID-19 pandemic [3].
The distribution of wind energy varies with altitude. The generic height of wind turbines is 80 m where wind speeds are greater than 6.5 m/s [21]. High speeds are crucial as power is proportional to the cube of the velocity. From Betz Law, the theoretical maximum efficiency of a turbine is approximately 59% with most turbines being able to extract only 50% of the wind energy that passes through the rotor [22]. The mechanical energy of the wind turbine is fed to the generator through a gear box that helps maintain the same speed for the synchronous generator. The output can then be fed into a Matrix or Z-source power converter. Most modern wind turbines have horizontal axis with three rotors placed up wind of the nacelle, which is the main body that contains the gearbox, mechanical brake, control systems, yaw drive and generator.
Wind turbines can either be designed to operate at variable or fixed speeds. More effective power capture, up to 8-15%, is produced with variable speed generators as well as being more cost-effective, noise-reduced and design-lighted [23]. As shown in Figure 6, a synchronous generator produces variable frequency AC power that is later transformed into a fixed frequency AC power to ensure a smooth grid connection.
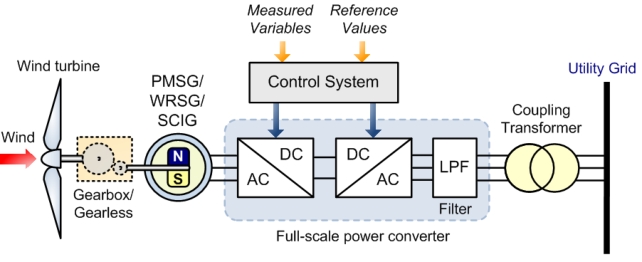
Figure 6. Direct In-Line Full Variable Speed-Controlled Wind Turbine with the Generator Connected to the Electrical Grid through a Full-Scale Power Converter. Reprinted with permission from [24].
The two main categories for wind power in electricity generation are offshore and onshore. Onshore wind is the more established approach, holding a 95% share of the current global installed capacity of wind. However, European energy policies have driven an uptake in offshore projects, with 26 offshore wind farms currently under construction [25]. Greater average wind speeds and a more regular supply are two significant advantages of offshore wind, as these lead to higher capacity factors and hence greater utilisation of the available wind energy. The capacity factor is a measure of the reliability of an energy resource, describing how much its output can be trusted. While the capital and operating costs for offshore wind are currently higher than onshore alternatives, the offshore potential energy generation is much greater [26].
In 2019, predictions indicate that offshore wind could provide 420,000 TWh annually – 18 times greater than the current global electricity demand [27]. Offshore farms allow for higher capacity wind turbines to be built, as there is no space constraint. This enables a larger rotor diameter for each turbine, increasing the swept area and therefore capturing more wind through which electricity can be generated as shown in Figure 7 [29]. The huge offshore potential could be further exploited through the recent development of floating wind farms to remove the constraint of having to build farms close to shores where the seabed is shallow, allowing higher and steadier windspeeds at greater ocean depths to be accessed. It is estimated that 80% of the offshore wind potential could lie at ocean depths greater than 60 m, which would only be accessible through floating farms [29]. This concept has been explored further by Lathigara et al. [30].

Figure 7. Comparison of Offshore and Onshore Wind Turbines.
The UK is the current world leader in offshore wind power generation, with the most installed capacity of any country and ambitious plans to power every home through offshore wind by 2030 [31]. This is made possible by major offshore projects such as the Hornsea One wind farm whose1.2 GW capacity makes it the largest in the world [32]. As well as powering one million UK homes, the electricity produced is being used to generate renewable hydrogen through the Gigastack project. As part of this project, water is decomposed into hydrogen and oxygen through electrolysis, enabling carbon-free hydrogen to be collected [33]. This forms a key part of the UK energy strategy going forward as hydrogen can be used to target hard-to-abate sectors such as the transport and industry, as explored further by Chapman et al [34].
2.3. Solar Energy
Much like wind energy generation, the deployment of solar energy generation is rapidly increasing, driven by policy incentives and the pursuit of decarbonisation. Favourable economics and subsides have increased interest of public investment into rooftop solar Photo-Voltaic systems, effectively eliminating demands for electricity from the grid.
The solar panels comprise of many smaller photovoltaic cells, arranged in series, that are made of two semi-conductive layers, typically silicon or germanium (Figure 8). Each individual solar cell acts as a PN junction. The oppositely charged layers create an electric field and as photon particles hit the surface, the cell becomes energised causing freed electrons to shuttle in the circuit, creating an electrical current [35]. For power appliances, an inverter is used for the conversion from DC to AC with any surplus AC going back to the grid.
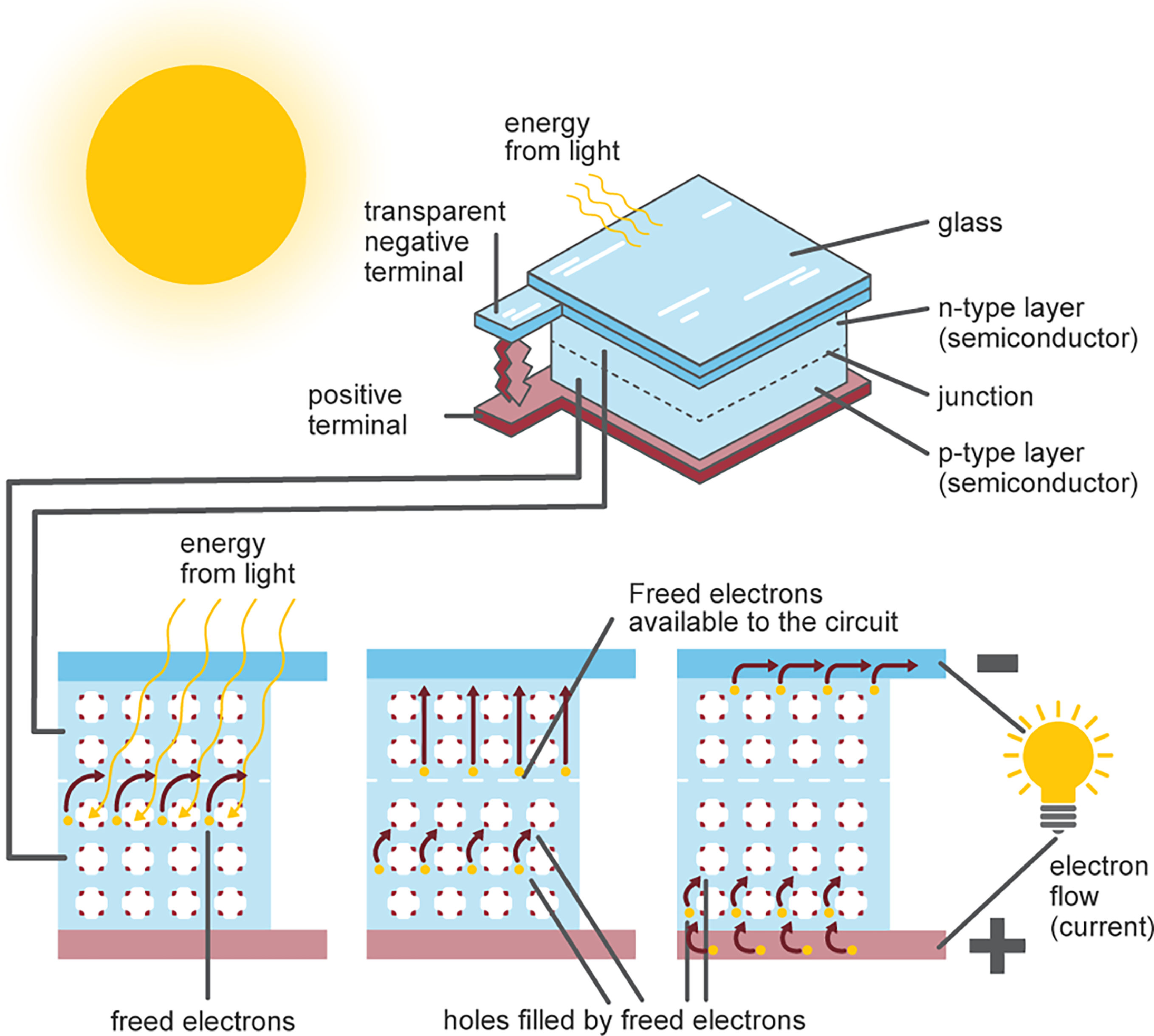
Figure 8. Diagram of Photovoltaic Cell. Reprinted with permission from [36].
The two main types of solar energy technologies are photovoltaic (PV) and solar-thermal. The PV technology is used to directly convert solar irradiation into electricity. PV cells are made from semi-conductor materials, and each individual cell with standard size of 156 mm by 156 mm can produce 1-2 W of power. Therefore, individual cells are connected in series and parallel to form solar modules with a greater overall power output. Modules can then be connected to form solar arrays which are used to supply electricity to the grid, or for distributed generation purposes [13]. Solar-thermal technologies capture heat energy directly from solar irradiation, which can then be used to meet consumer heat demand through space and water heating appliances. In 2019, 36% of UK gas consumption was attributed to domestic use, predominantly for space and water heating [37]. The increased use of solar thermal as a low-carbon substitute for gas heating could therefore significantly reduce gas consumption and accelerate decarbonisation [38].
From 2010 to 2020, the cost of producing solar energy using utility-scale solar photovoltaics (PV) has dropped by 85% [40]. The global installed capacity of solar photovoltaic (PV) power in 2021 was 760 GW, with an additional 139 GW being installed in 2020. This surpassed the growth of wind power in 2020 which saw only a 93 GW increase in installed capacity [3]. With the significant growth of installed solar energy capacity, the International Energy Agency (IEA) predicted an installed capacity of 1830 GW by 2026 [40], which is 2.4 times of that in 2021. According to the International Renewable Energy Agency (IRENA), the solar energy capacity has the potential to increase to 8519 GW (11.2 times of that in 2021) by 2050 to meet the targets set out in the Paris Climate Agreement of 2015. The current installed capacity is shown in Figure 9 alongside the future projections.
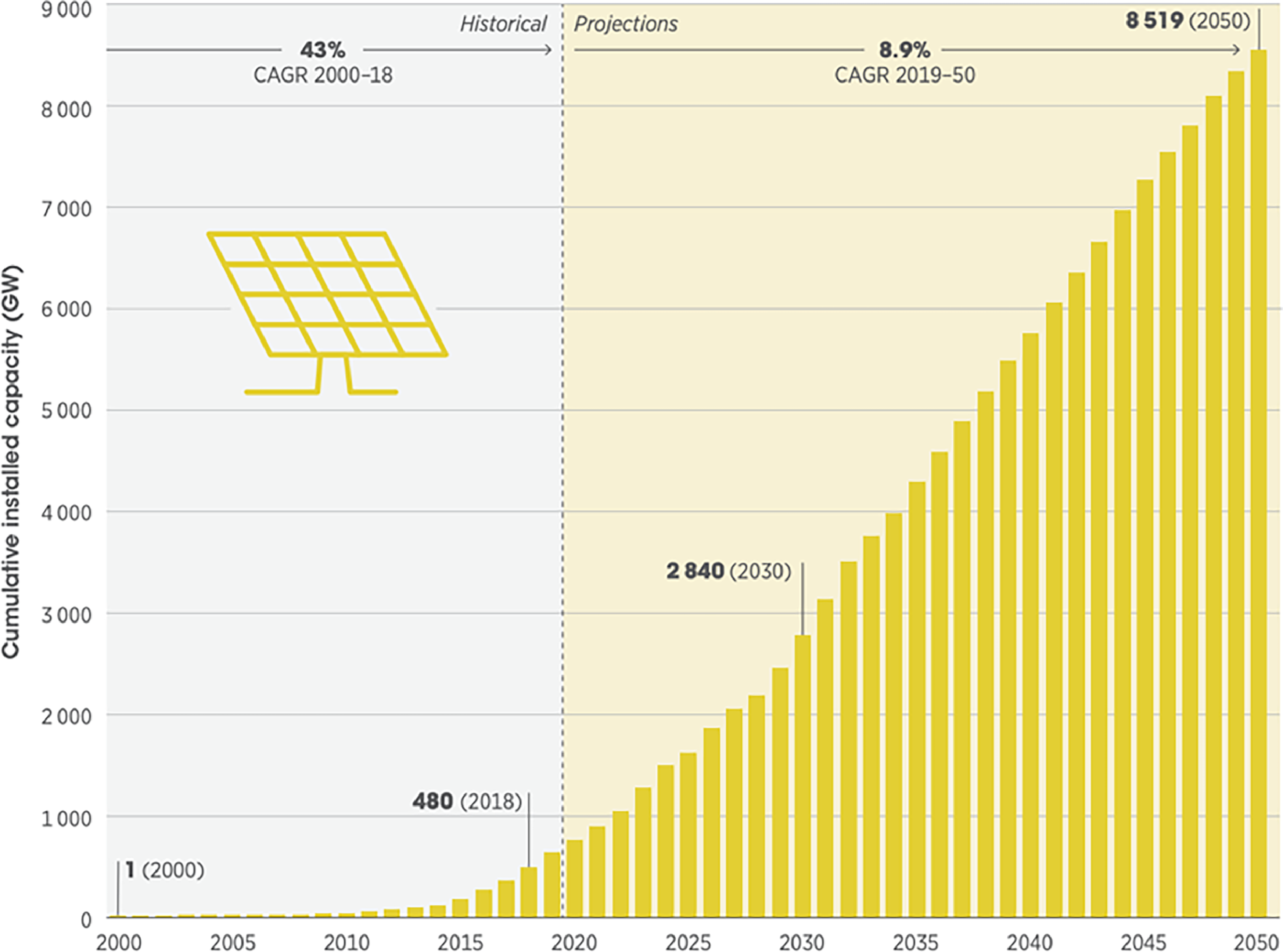
Figure 9. Solar power installed capacity projections. Reprinted with permission from [39].
2.4. Development and Applications
Due to the significant growth rate of renewable energy generation, the proportion of renewable electricity in global electricity increased from 20.3% in 2011 to 28.5% in 2021, and it is predicted to achieve 60% in 2030 (Figure 10) [8]. Following the IEA 1.5 degree pathway until 2030, total renewable electricity will increase by about 3 times, with solar energy increasing by 6.8 times and wind energy by 4.3 times [8].

Figure 10. 2030 Renewable Electricity Share Prediction.
Although renewable energy is developing at a tremendous speed, the amount of usage is significantly lower than its availability. There are three main application sectors where renewable energy can make a substantial difference: buildings, industry & agriculture, and transport. Based on data in 2019, the proportion of renewable energy usage is 14.7% in buildings, 16.1% in industry & agriculture, and only 3.7% in transport [42]. The proportion of renewable electricity was 26.63% in 2019, but the renewable energy usage was only 19.32%, shown in Figure 11. One of the reasons behind the limited amount of renewable energy usage is the storage limitation. Renewable energy could be wasted if there is no effective storage, and the energy stored will also be discounted due to storage efficiency. The inability to store and send power when required is regarded as one of the primary drawbacks of renewable energy [43].
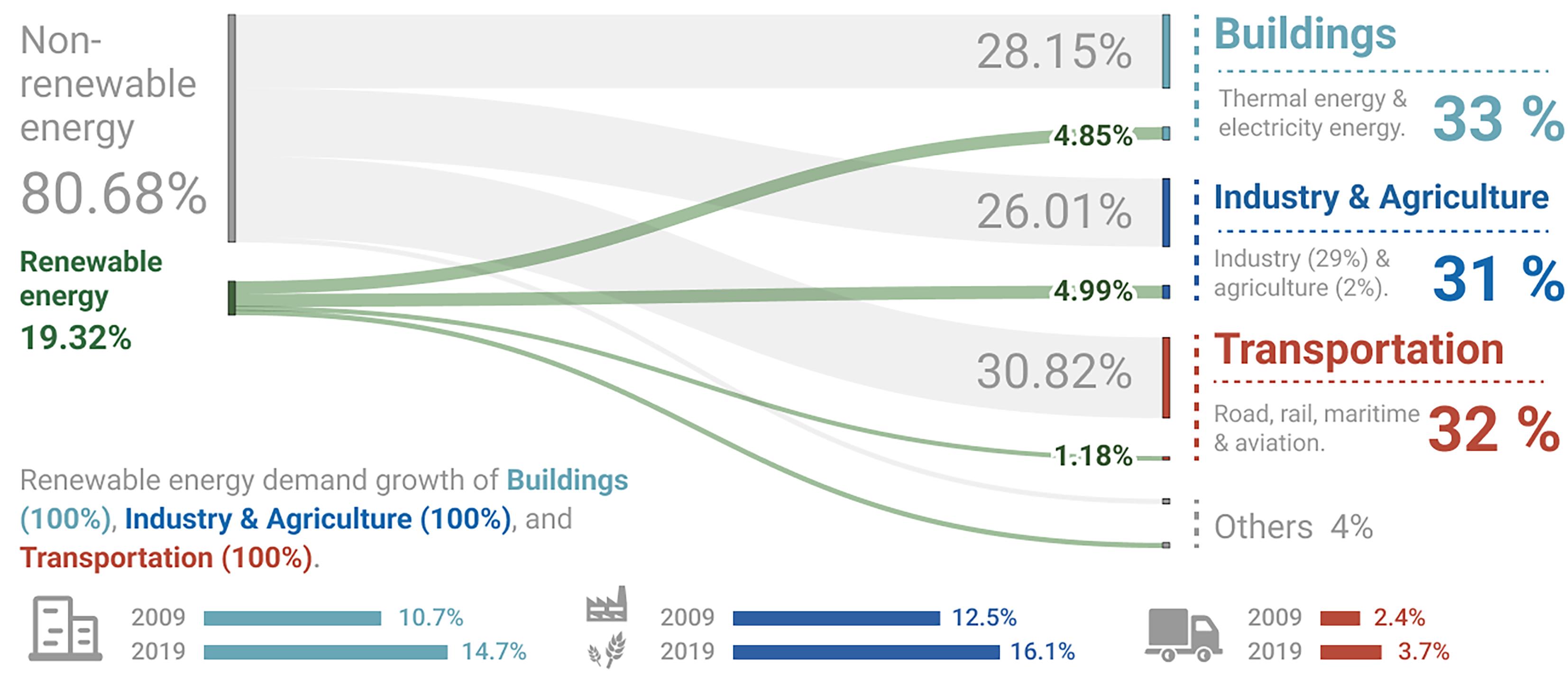
Figure 11. 2019 Renewable Energy Usage in Buildings, Industry & Agriculture and Transportation.
Figure 12 shows a more detailed composition of energy usage in transport. From 2009 to 2019, the share of renewables used in transport increased from 2.4% to 3.7%, but the usage of renewable electricity increased from 0.2% to only 0.4%. By the end of 2019, 99.6% of transports are still using internal combustion engines [42]. With encouragement of government policies and customer preferences, the EV sales tripled between 2019 and 2021 [44], However, fuels still have higher power densities than that of batteries, and a more effective energy storage technology needs to be developed to replace conventional internal combustion engines for more extensive renewable electricity usage in the transport sector.
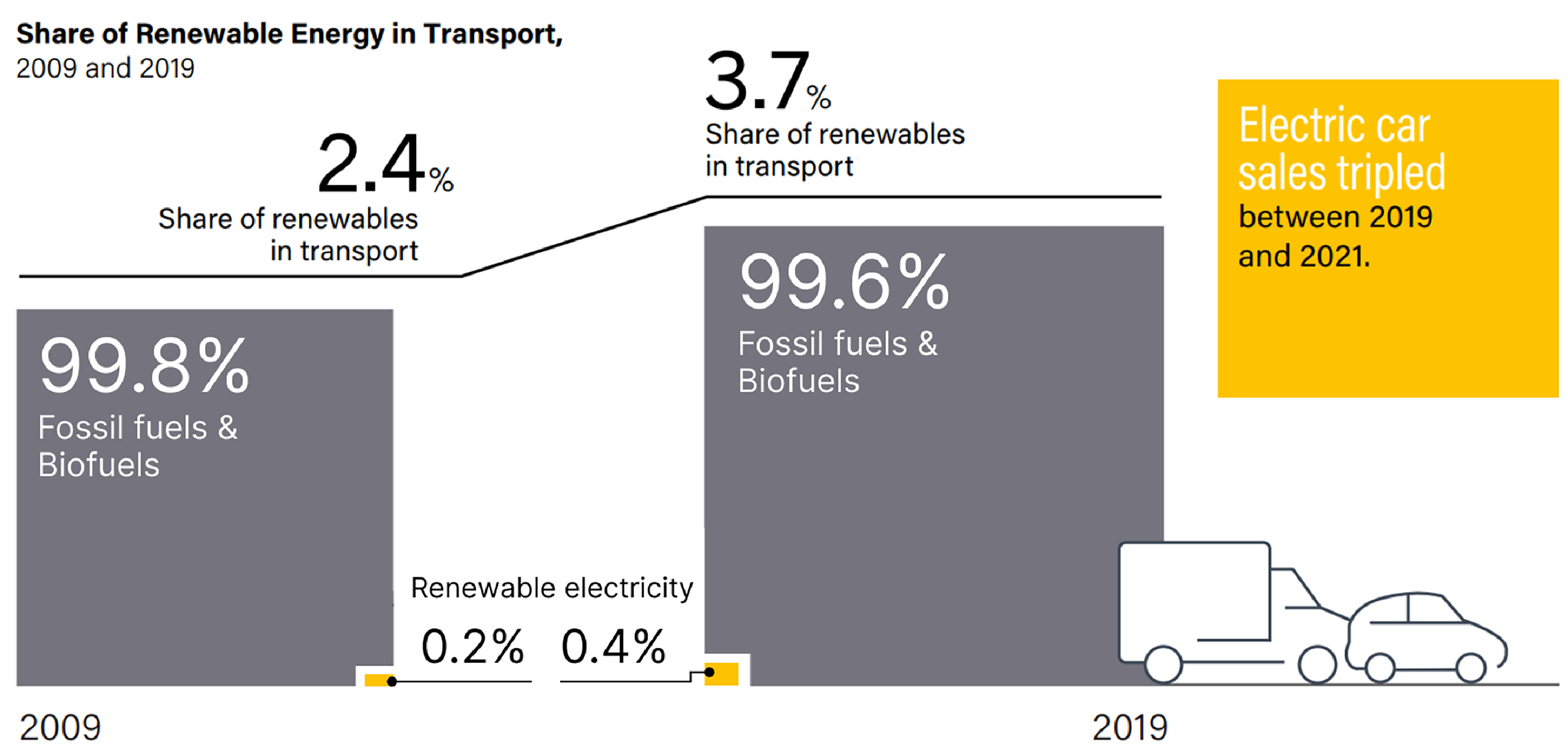
Figure 12. Share of Renewable Energy in Transport from 2009 to 2019. Reprinted with permission from [43].
2.5. Raw Material Demand for Renewable Energy Sources
The development of renewable energy is also accompanied with raw material cost for building individual operating systems. Figure 13 shows the raw materials requirements for building 1 MW solar PV plants, 50 MW onshore wind plants, and 500 MW offshore wind plants.
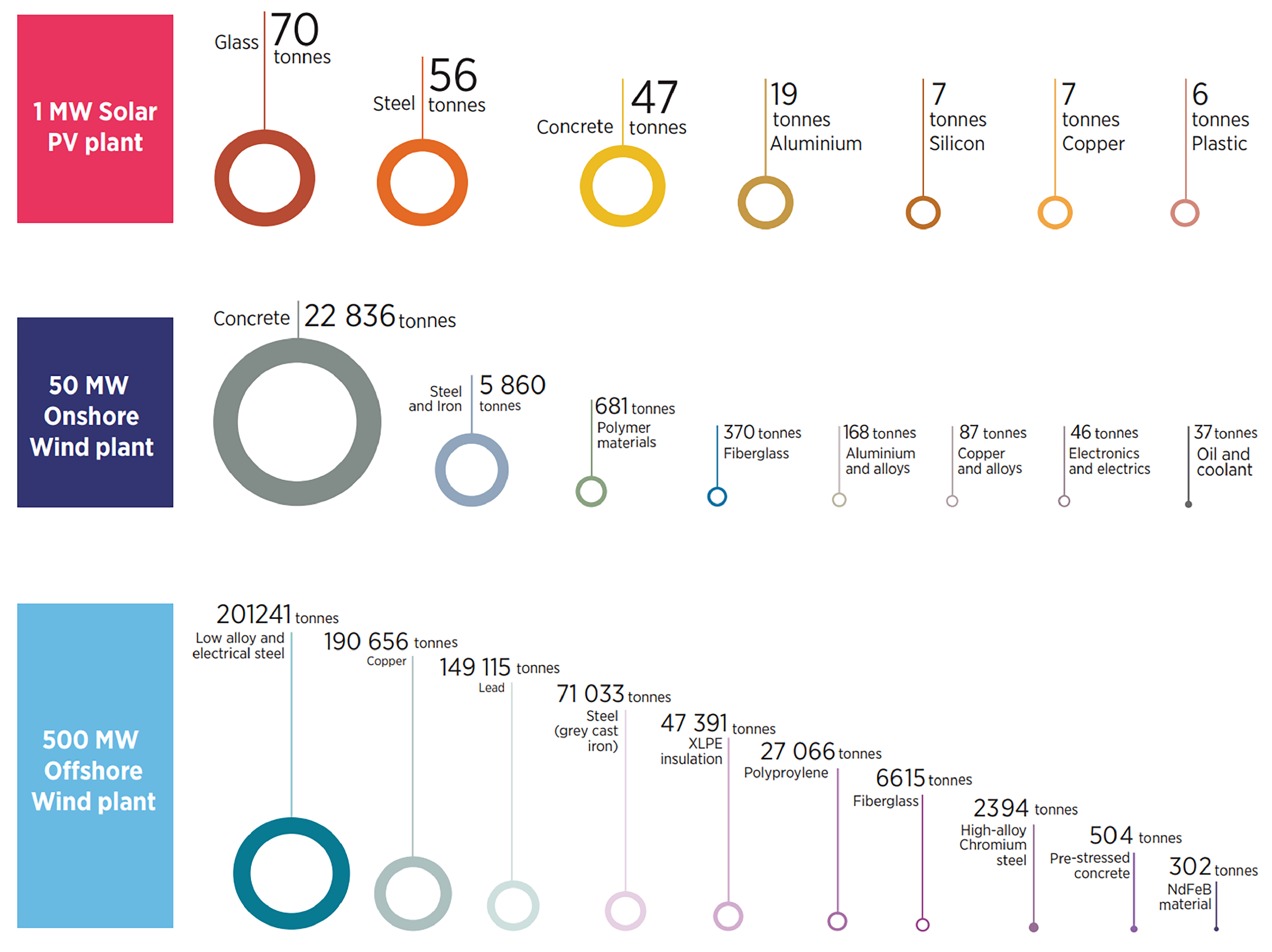
Figure 13. Raw Materials Breakdown for Building 1 MW Solar PV Plant, 50 MW Onshore Wind Plant, and 500 MW Offshore Wind Plant. Reprinted with permission from [45].
Although the renewable energy was developed for environmental-friendly purposes, the number and variety of raw material used in building power plants are larger than expected.
3. Overview of Renewable Energy Storage Technologies
Energy storage is set to be an important technology in the cost-effective integration of renewable energy resources for the automotive applications. In this section, we highlight the widely used centralized energy storage (including different types of rechargeable batteries and hydrogen fuel cells) and prospective distributed energy storage realized by the embodied energy storage technology.
3.1. Centralized Energy Storage Technologies Realized by Rechargeable Batteries
With global EV sales dramatically growing in the past years (Figure 14) [46], environmentally friendly rechargeable batteries employed in EVs have attracted tremendous attention around the world. The EVs convert chemical energy stored in their active materials into electrical energy, through reduction and oxidation reactions occurring at the interfaces between the electrode and the electrolyte.
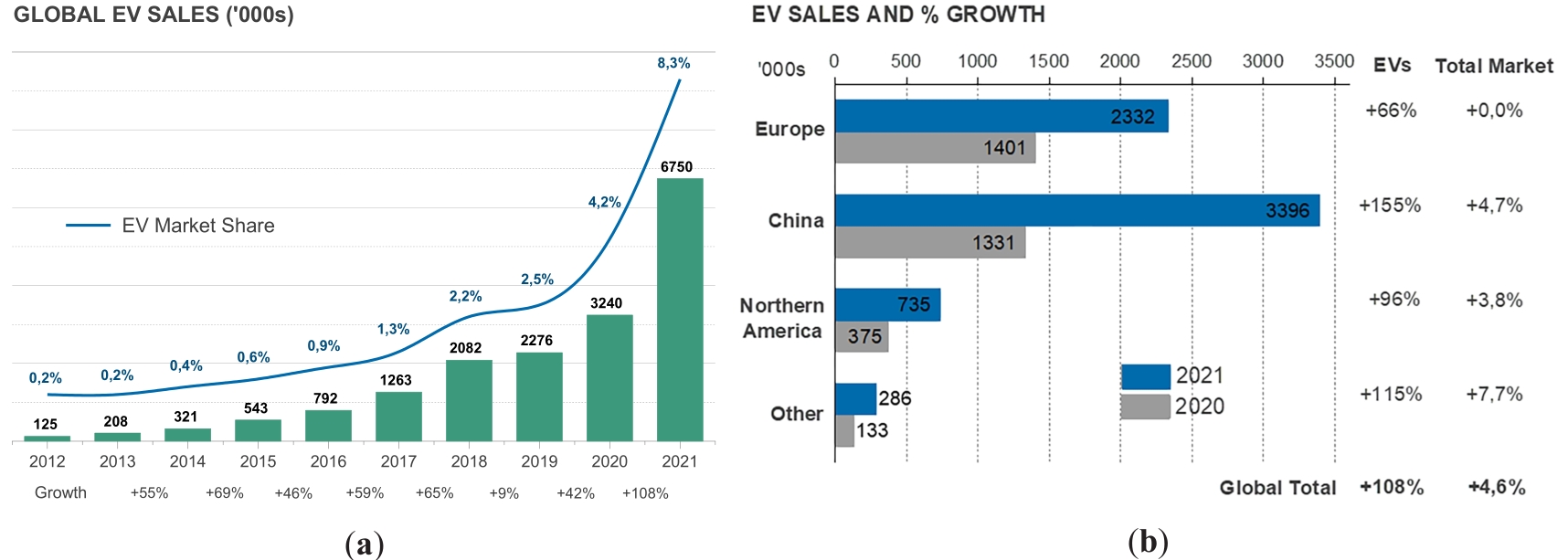
Figure 14(a) Global EV Sales and Growth in the Past Decade and (b) EV Sales and Growth in Europe, China, Northern America, and Other Worldwide Areas in 2020 and 2021. Reprinted with permission from [46].
3.1.1. Lead Acid (Pb-acid) Batteries
The Pb-acid battery composes of lead oxide as a cathode, lead as an anode, and sulphuric acid as an electrolyte [47]. It was introduced in 1859 and has become the most used and cost-effective rechargeable battery around the world. Moreover, it shows high inherent safety [48], high electrochemical activity, long cycle life [49], low-temperature operation [50], and low self-discharge rates. However, the low energy density (30-50 Wh/kg) owe to the high weight of lead impedes the further development of Pb-batteries in EVs.
3.1.2. Nickel-metal Hydride (Ni-MH) Batteries
In the EV market, the Ni-MH battery is the only contender in the group of alkaline batteries, in which the alkaline solution serves as an electrolyte. Nickel-cadmium (Ni-Cd) battery has been eliminated by the EV market because of the environmental issues caused by toxic metal cadmium, lower energy density (~50 Wh/kg) and high cost. Nickel-zinc (Ni-Zn) battery also exhibits severe problems such as the fast growth of dendrites, leading to battery failure. In comparison, since its commercialization in 1989, the Ni-MH battery with higher energy density (60-120 Wh/kg) and lower cost has become a substitute for other alkaline batteries and has been applied in hybrid vehicle propulsion systems [51].
A Ni-MH battery composes of metal hydride (MH) as a negative electrode, nickel hydroxide as a positive electrode and KOH as an alkaline electrolyte, allowing hydroxide ions to shuttle between the MH negative electrode and nickel hydroxide positive electrode [52]. In the Ni-MH battery, the alkaline electrolyte and lower-reactivity metals enable safety in operation at high voltage [53,54]. Moreover, Ni-MH battery has high power density, environmental compatibility, tolerance to overcharge and overdischarge, long cycle life and better low-temperature performance which enables EVs in cold climates [55,56]. However, there still exist several problems such as high self-discharge and relatively low capacity compared to LIBs, hampering the development of Ni-MH batteries in the EV.
3.1.3. Lithium-ion Batteries (LIBs)
Compared with other rechargeable batteries (Figure 15), lightweight LIBs with high energy density (100-265 Wh/kg), stable cycling performance and long cycle life [57,58] are currently considered one of the most ideal power batteries used in EVs.
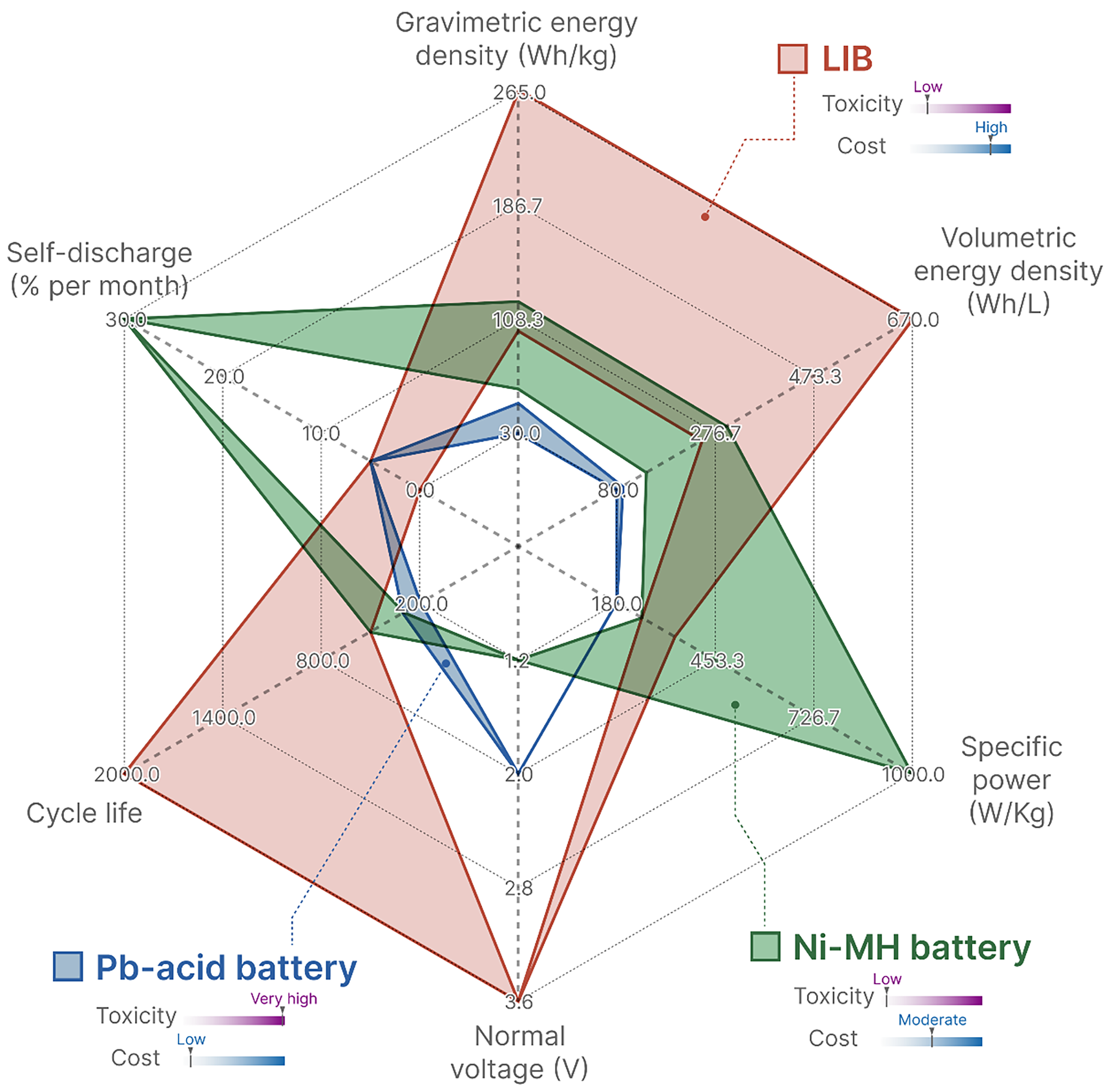
Figure 15. Comparison of Different Rechargeable Batteries.
The primary components for a LIB involve the electrodes (cathode and anode), electrolyte, separator, and packaging. For most LIBs, the cathode is composed of a positive current collector (aluminium foil) with lithium-oxide active materials coated on, while the anode is composed of a negative current collector (copper foil) with graphite-based active material coated on. To keep the cathode and anode in close proximity while fully separated to prevent short-circuiting, there is usually a separator soaked in a lithium-ion (Li+) conductive liquid electrolyte in between. Aluminium alloy, stainless steel or aluminium plastic seal are usually used as packaging. These components should be rationally assembled into a whole battery according to the basic principle of battery structural design. The structure of an LIB in a schematic diagram is shown in Figure 16. During the charging process, Li+ de-intercalating from the cathode material transfers through the electrolyte to reach and intercalate into the anode material, while the electrons transfer from the cathode to the anode via an external circuit. When discharging, the movements of Li+ and electrons are reversed. Cathode materials for conventional LIBs used in EVs are usually lithium nickel-rich oxide (NCM) and lithium iron phosphate (LFP), which can be charged to higher voltage when Li+ is removed [59,60]. Anode materials used in commercial LIBs involve graphite material and graphite incorporating silicon-based materials. Figure 17 shows the comparison between NCM and LFP LIBs in terms of specific energy, specific power, cycle life, safety, cost and voltage. The NCM LIBs with high energy density due to high voltage and high cost due to the scare of cobalt and nickel, are expected to be used in high-end performance EVs. While LFP LIBs with lower cost and specific energy are expected to be used in buses and delivery trucks.
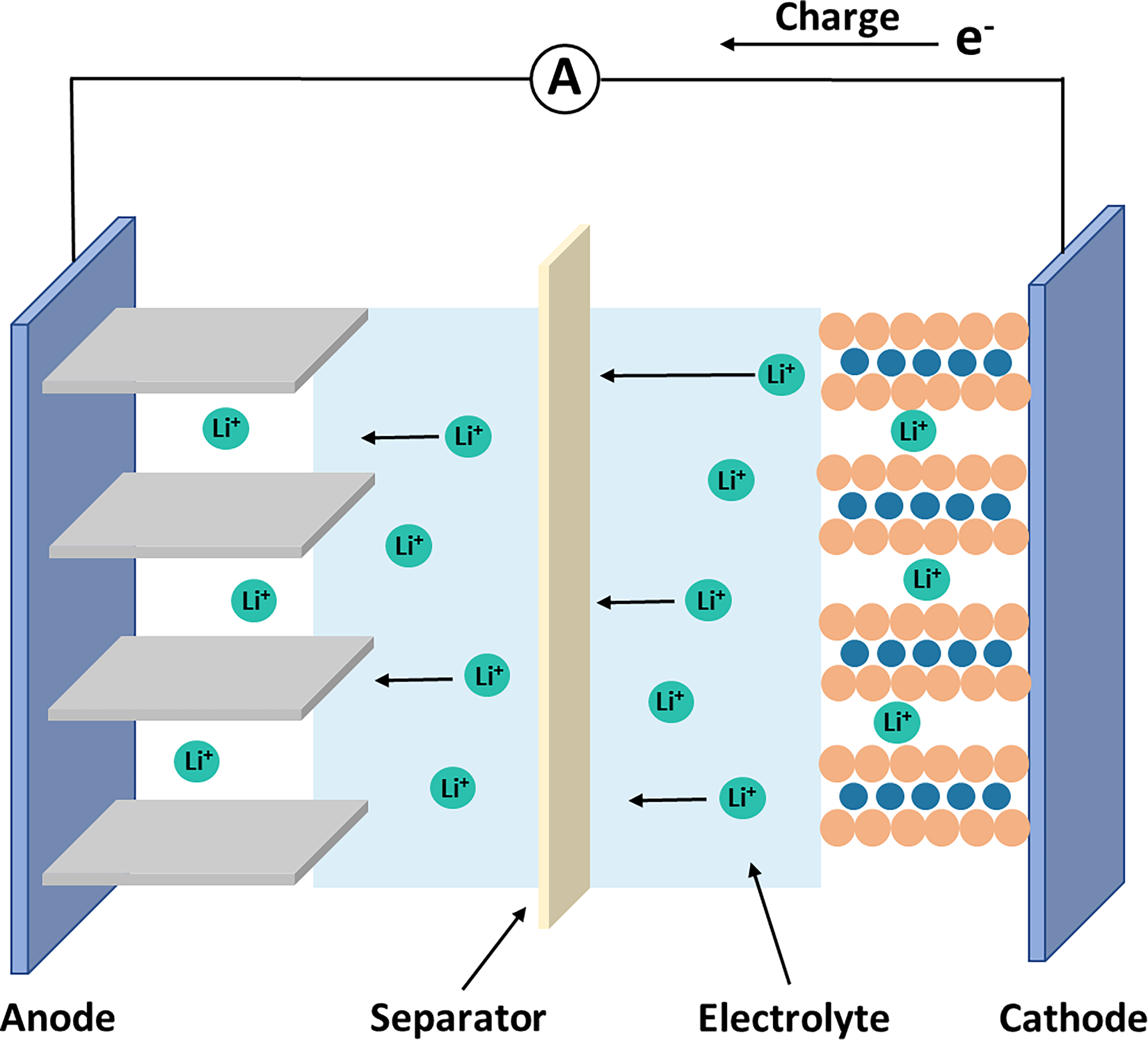
Figure 16. Schematic of Charging Process in lithium-ion batteries (LIBs).
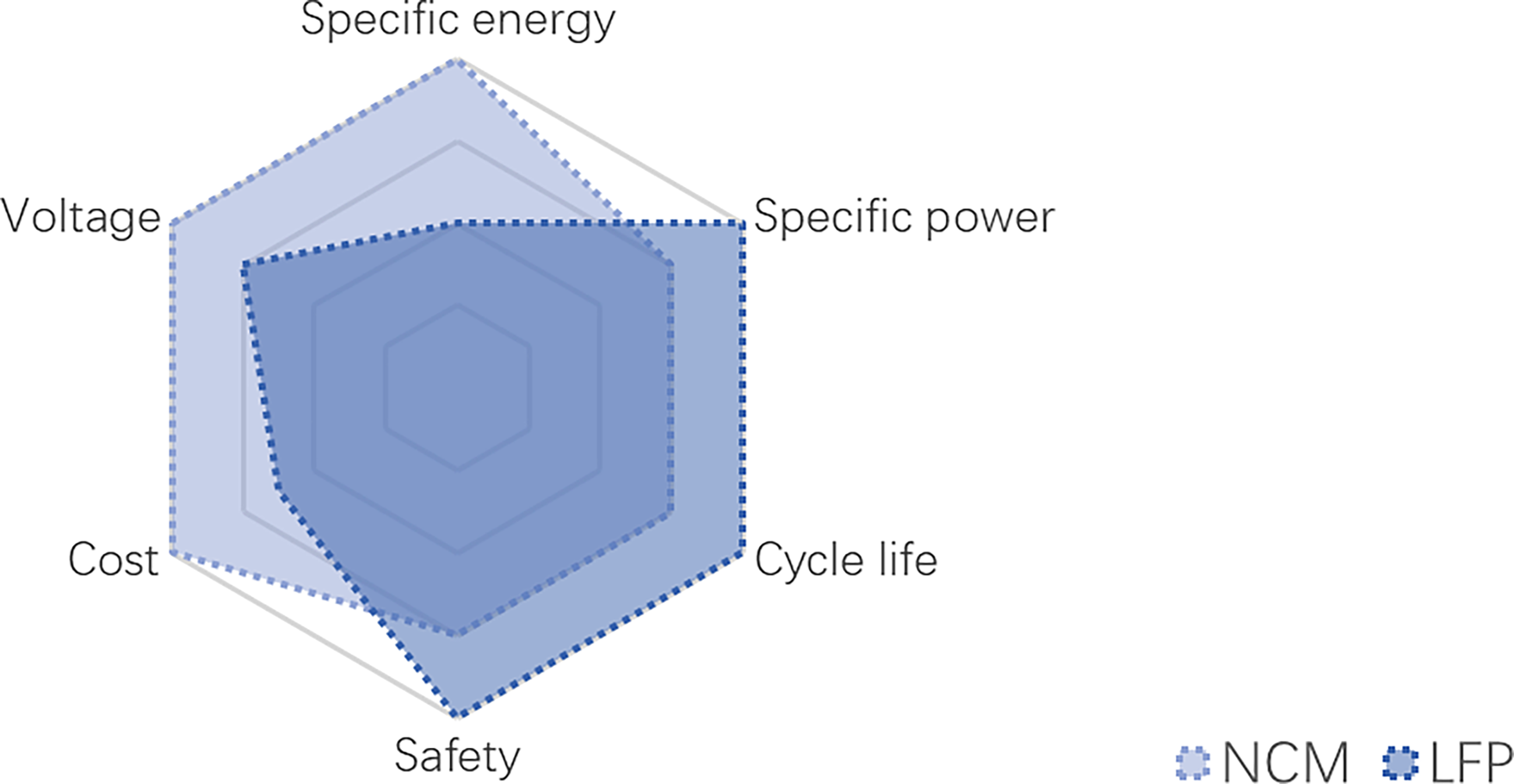
Figure 17. Comparison of nickel-rich oxide (NCM) and lithium iron phosphate (LFP) LIBs.
Regarding physical implementation, conventional LIBs used in EVs are generally categorized into three typical formats, including pouch cells, cylindrical cells, and prismatic cells (Figure 18). Pouch cells with a relatively high specific energy, cycling performance and safety have been growing in popularity for EV applications in recent years, and generally pouch cells are costly and cannot be mass-produced on a large scale. The cylindrical cell with a high capacity, a long cycle life and a low cost is the most commonly used LIB format in EVs. However, the cylindrical cells cannot be densely stacked owing to space cavities, thus occupying more space in EVs. Prismatic cells possess a thinner size but are heavy, bulky, and concentrated, which delivers negative impact on the driving range, storage space and dynamic handling. All these conventional LIBs remain with lots of accessories, increasing the inactive weight. Moreover, LIBs as centralized monofunctional energy supplies remain isolated from the system. According to the statistics, the LIB pack takes up nearly 30% of the total weight of a typical EV of TESLA MODEL S, while the fuel only takes up approximately 3% in a petrol-run car of HONDA CIVIC, as shown in Figure 19 [61,62]. Therefore, there is a urgent demand urngent to explore lightweight and efficient battery energy storage systems that can be used in EVs a.

Figure 18. Three Typical Formats of LIBs.
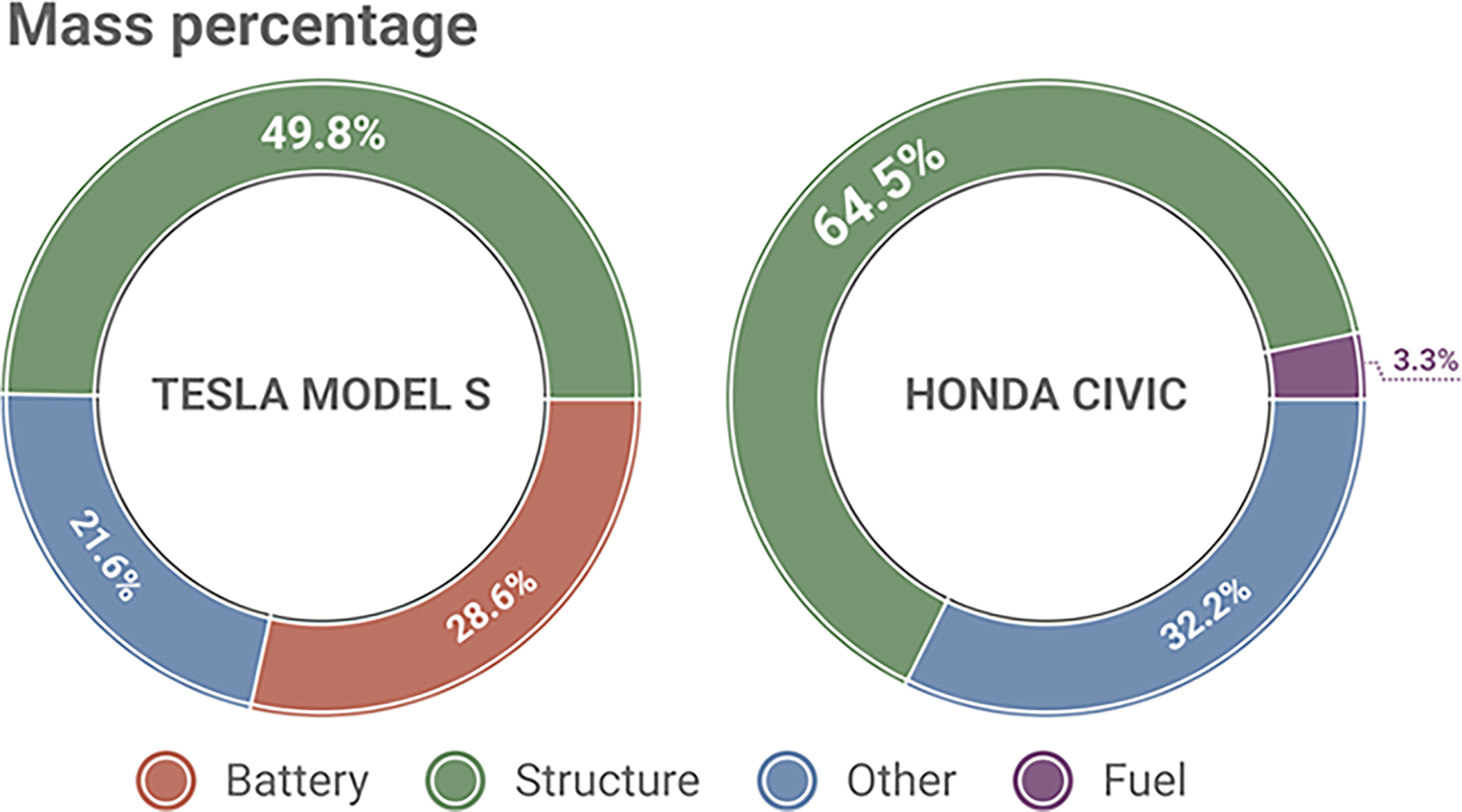
Figure 19. The Comparison between the Mass Share of Energy Sources in an EV of TESLA MODEL S and a Petrol Car of HONDA CIVIC.
It is worth mentioning that here have been only very few improvements over the past decade on LIBs, although extensive work has been performed on LIBs to minimize inactive weight [63], simplify fabrication procedures by using full-automatic assembly line, including coating, stacking, welding and enclosing processes [64,65] and optimize packaging by using metal case with higher mechanical properties [66,67].
3.1.4. Solid-state Lithium-metal (Li-metal) Batteries
Li-metal batteries are considered the next generation high energy density LIBs for EVs due to the high specific capacity (3680 mAh/g) and low electrochemical potential of Li-metal anode [68]. However, the overgrowth of Li dendrites on Li-metal anode easily penetrates the separator and thus leads to short circuits. To solve the safety issues caused by using the Li-metal anode and conventional liquid organic electrolyte with low thermal stability and low flame point [69–71], solid-state electrolytes with high mechanical and non-flammable properties are expected to be applied in EVs in the future. The solid-state Li-metal battery would significantly improve the energy density and at the same time, reduce the fire accidents and explosion of EVs.
Solid-state electrolytes serve as the ion transport medium allowing efficient shuttling of Li ions between the cathode and anode, while preventing short circuit between two electrodes during charge-discharge cycling. So far, a wide range of solid-state electrolytes, including solid oxide electrolytes (e.g. NASICON-type [72,71], garnet-type [74,75] perovskite-type [76,77] et al.), solid sulfide electrolytes, solid polymer electrolytes (SPEs) such as poly (ethylene oxide) (PEO)-based electrolytes and poly (vinylidene fluoride) (PVDF)-based electrolytes, are being intensively studied [78–81].
The solid-state Li-metal battery shows great improvements in safety, mechanical properties and energy density compared to traditional liquid LIBs, and thus is promising to be used in EVs in the future. However, the low ionic conductivity of the solid-state Li-metal battery at the room temperature (10-7-10-4 S/cm) fails to meet the practical utilization requirement.
3.2. Centralized Energy Storage Technologies Realized by Hydrogen Fuel Cells
The fuel cell is an electrochemical device that efficiently produces electricity from the chemical energy of hydrogens and other fuels by a pair of redox reactions. The schematic of a hydrogen fuel cell was shown in Figure 20. Hydrogen fuel cells have great potential for decarbonisation in the automotive industry since hydrogen can be produced from renewable energy sources [82,83], but (1) the products in a hydrogen fuel cell (including electricity, water, and small amounts of heat) are easy to cause negligible environmental impact; and (2) the high cost of components in hydrogen fuel cells, including humidifiers, costly platinum catalyst, and heat exchangers, hinders possible commercial advancement [84,85]. Additionally, the challenge of hydrogen storage, transportation, and distribution is another barrier to the vast applications of hydrogen fuel cells in automotive manufacturing [86]. Consequently, more research efforts need to be devoted to addressing these challenges in the future.
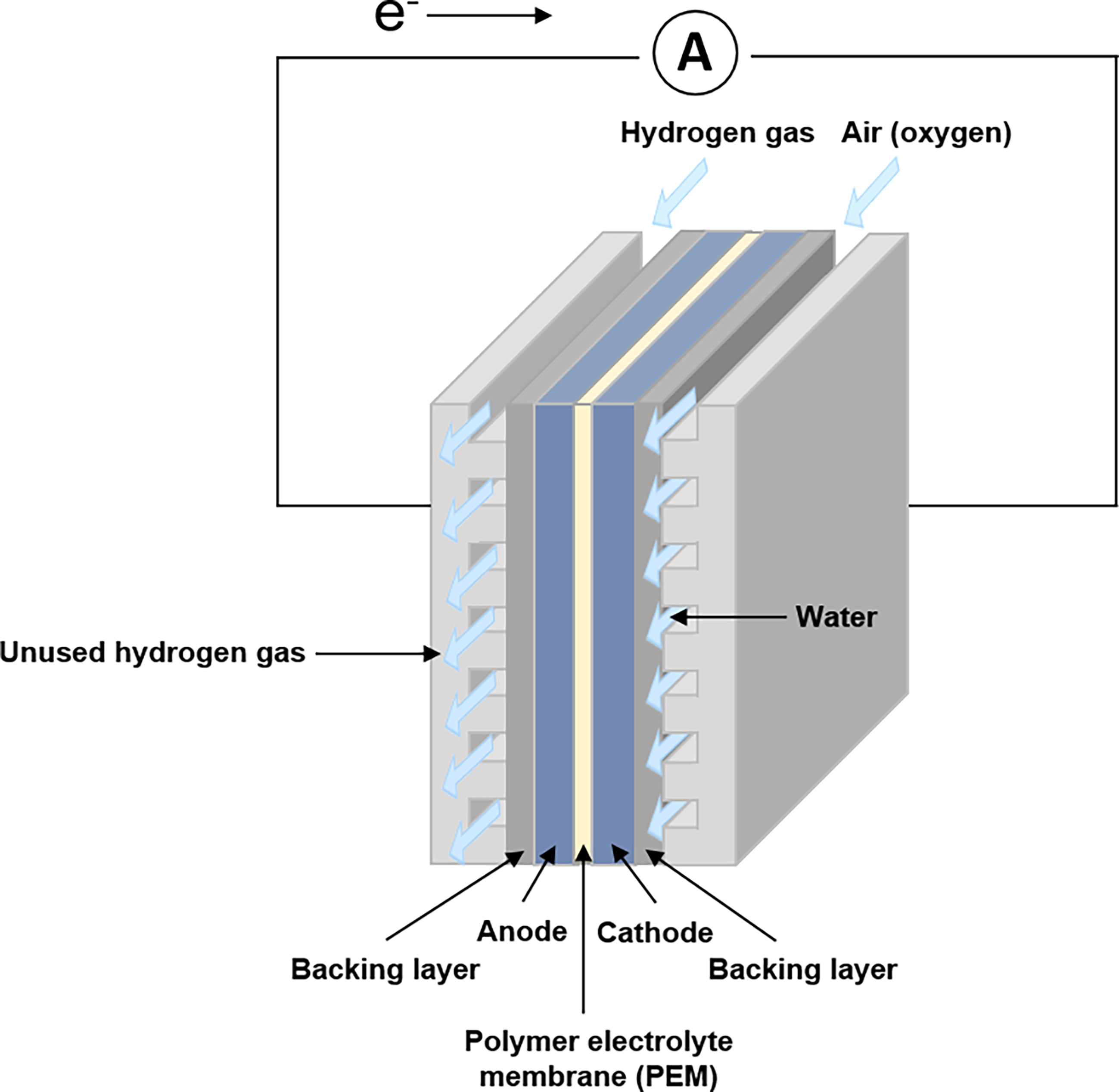
Figure 20. Schematic of Hydrogen Fuel Cell.
3.3. Summary: Various Centralized Energy Storage Technologies for Automotive Applications Are Non-substitutional
The rechargeable batteries and hydrogen fuel cells with enhanced energy density, power density, safety and reduced cost enable the long driving range and cost-effective zero-emission automotive applications. LIBs with high energy density and long cycle life have been widely used in EVs, while the fast-refuelling hydrogen fuel cell can be an alternative solution to battery EVs in terms of long range applications. When it comes to transportation needs exceeding a range of 300/350 miles, battery EV is not a viable choice due to its short driving range and the long time for battery charging, whereas the hydrogen fuel cells can offer a larger range because vehicles can store more hydrogen without a significant weight and cargo volume penalty. Therefore, fuel cells could be the main choice for long-distance transportation, whereas battery EVs will be focused on short range commercial vehicle deliveries and passenger vehicles.
In conclusion, as the energy storage technologies for automotive applications, both the rechargeable batteries and hydrogen fuel cells are not substitutional. Therefore, investment in transportation applications needs to be balanced according to the adoption of each technology in different conditions.
3.4. Distributed Energy Storage Realized by Embodied Energy Storage Technologies
Inspired by the requirement of lightweight production in EVs, distributed embodied energy storage technologies are being intensively explored. Embodied energy storage can be achieved by introducing structural power batteries and supercapacitors, which simultaneously enable electrical energy storage function and mechanical function [87]. This would promote the energy storage system in EVs toward lightweight and volume-saving development. More specifically, as shown in Figure 21, the future EVs are expected to have electrical energy storage function in the body metal panels, e.g. the frames, roof, doors, chairs, and floor [88].
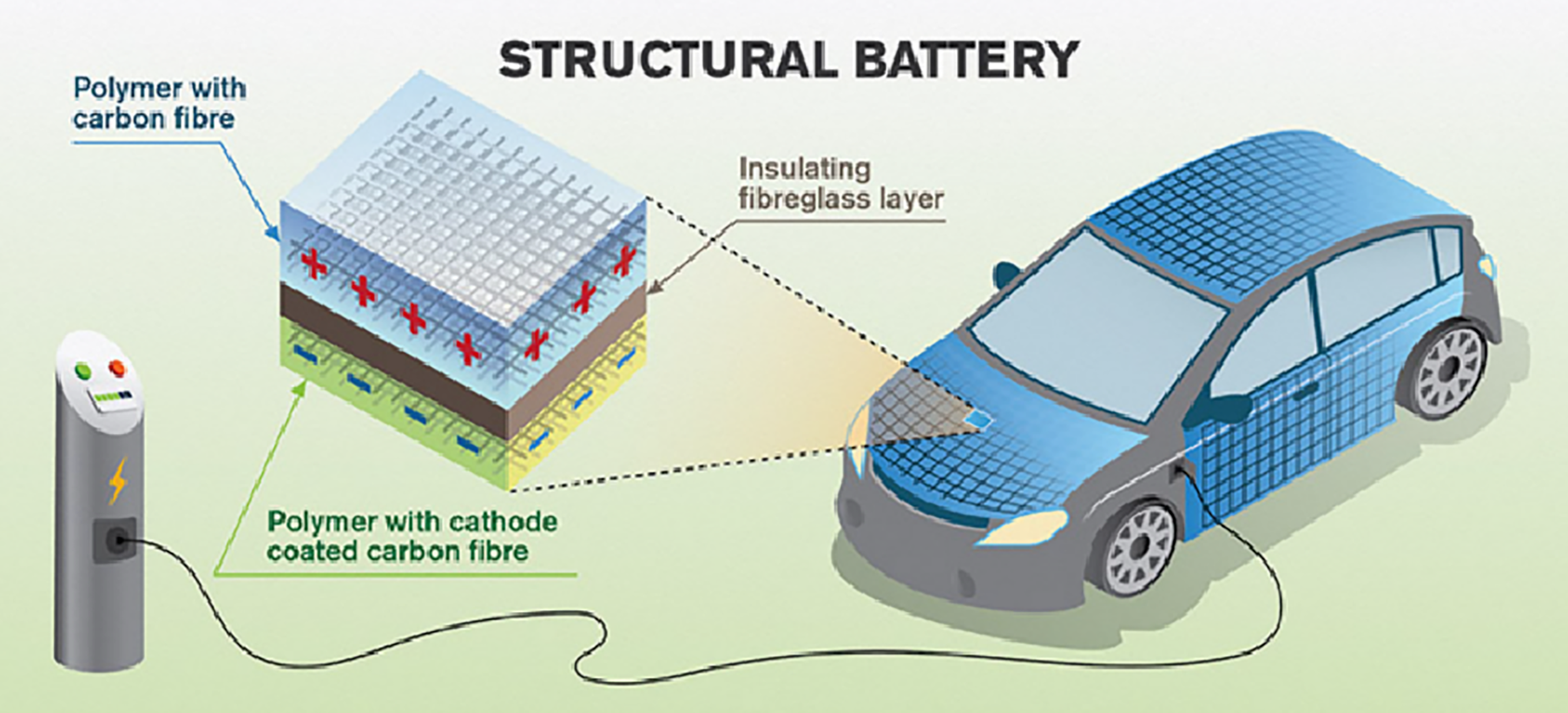
Figure 21. Structural Battery Used in an EV. Reprinted/adapted with permission from [88].
To date, two strategies have been reported to realize structural power batteries. One strategy is to embed a thin prismatic battery cell core into composite laminate skin panels (e.g. carbon fibre reinforced laminates) to assemble a sandwich structure. This strategy enables electrochemical energy storage in a structural component. Numerous structural power batteries have been assembled by following this strategy [89–92]. Zhang et al. [93] presented a multifunctional structural lithium-ion battery, using mesocarbon microbeads as the anode, LiNi1/3Co1/3Mn1/3O2 as the cathode and LiPF6 as the electrolyte, in a sandwich panel architecture. The figure demonstrated an energy density of 248 Wh/L with a capacity retention of 85.9% after 190 cycles at ~C/3 rate and three-point bending tests at a load up to 1060 N. Ladpli P et at. (93) developed a multifunctional energy storage composite (MESC) structure, in which the LIB was embedded in the carbon fibre composites by polymer rivets without any battery chemistry modifications, shown in Figure 22. Although the structural battery manufactured by this strategy exhibited higher energy density, it provides limited weight and volume reduction for the system, and this has inspired the development of multifunctional structural battery composite materials to enhance the potential for weight reduction.
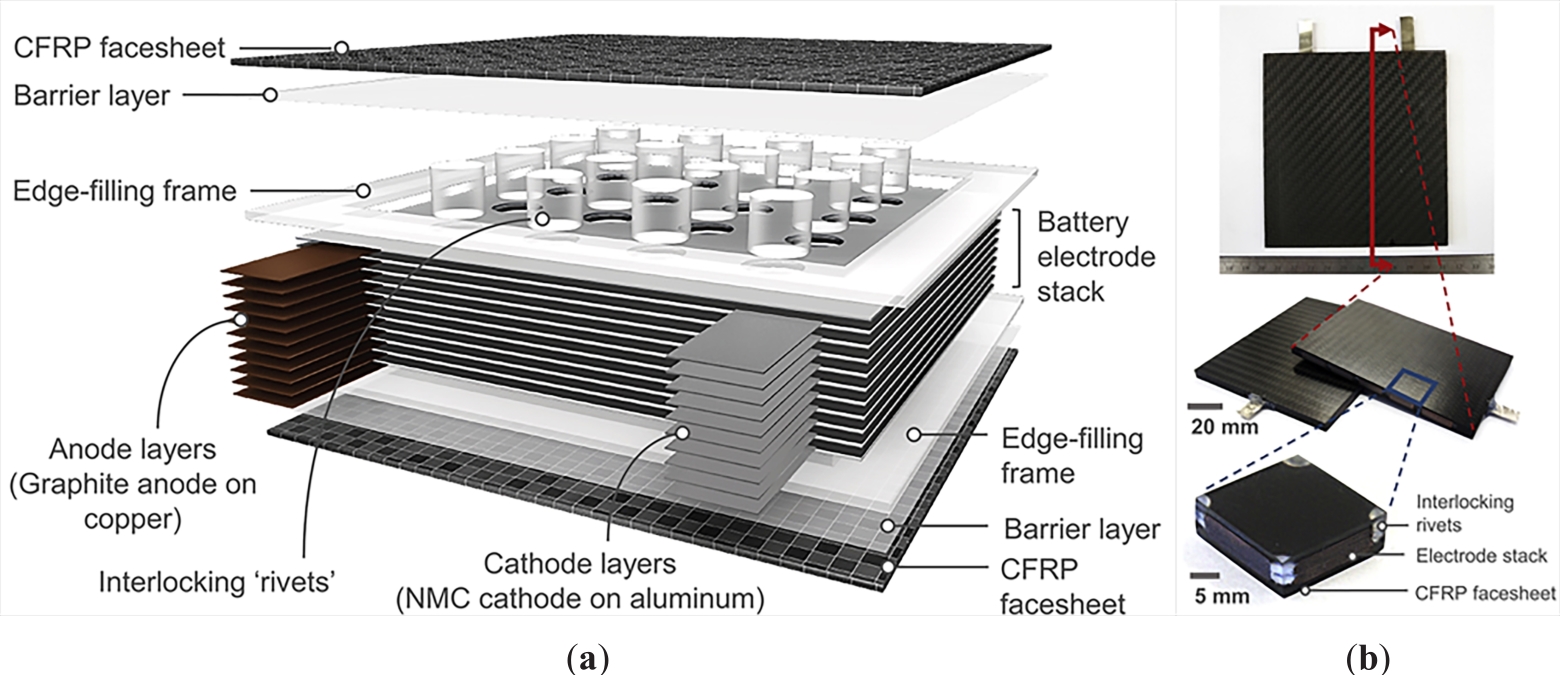
Figure 22(a) 3D schematic of MESC and (b) As-fabricated MESC coupon. Reprinted/adapted with permission from [93].
Developing multifunctional structural battery composite materials is an alternative strategy to achieve the structural power batteries, in which all constituents concurrently perform multiple roles, including structural electrode composites, structural separator composite and structural electrolyte composite [62,94–97]. In this case, the reinforcing carbon fibres employ as an electrode and the polymer composites serve as a matrix and electrolyte. The first structural battery composite material was designed by Wong et al. [98], i.e., the carbon fibre anode where LiFePO4 was deposited on a metal substrate as a cathode with a glass fibre separator and a solvent-free structural vinyl ester polymer electrolyte matrix employed in this design. Although the structural battery material delivered outstanding mechanical properties, it fails to be characterised by electrochemical tests as a result of poor electronic insulation. Later, Ekstedt et al. [99] fabricated the structural battery composite materials based on carbon and aluminium fibre weave electrodes, a glass fibre separator, and a gel electrolyte. This structural battery composed of LixC6 and LiFePO4 has an Open Cell Potential of 3.3 V, but was not able to provide matching mechanical and electrochemical capacities compared with the conventional polymer composite and solid polymer electrolyte-based LIB, respectively.
Asp et al. [94] demonstrated a structural battery composite, consisting of a LiFePO4 cathode and a carbon fibre (CF) anode separated by a glass fibre fabric separator in a structural battery electrolyte. It delivered an energy density of 24 Wh/kg at a current density of 0.05C with an elastic modulus of 25 GPa. To improve the electrochemical performance of the multifunctional structural battery, Moyer et al. [100] employed an electroconductive poly acrylonitrile (PAN) coated onto the surface of an LiFePO4 cathode and a graphite anode with the CF current collectors to assemble a full-cell structural LIB. As-prepared PAN reinforced interfaces enhanced the mechanical adherence and ion transportation between battery active materials and CF current collectors. As a result, this Li-ion structural battery presented capacity retention of 80.5% over 100 charge-discharge cycles at a cycling rate of 0.1 C and achieved an average gravimetric energy density of 52 Wh/kg, which is approximately twice higher than a structural battery without a PAN reinforced interface.
For practical applications of embodied energy storage in the automotive industry, Shaffer et al. [101] manufactured a structural supercapacitor by using carbon aerogel (CAG) modified structural carbon fibre (CF) fabric electrodes, which were separated by structural glass fibre fabric filled with a polymer electrolyte. A component of the car trunk lid was produced for a Volvo S80, in which LEDs were lightened by the structural supercapacitors (Figure 23). W Johannisson et al. [102] modelled and compared three roofs used in an EV made from mild steel, carbon fibre and epoxy composite, and a multifunctional structural battery. The roof made from the structural battery delivered a mass saving of 62% and 20% compared with the original steel roof and updated composite roof, respectively (Figure 24). Complementary, Asp et al. [103] presented three scenarios for Tesla Model S and the BMW i3, where the conventional monofunctional LIBs pack was fully or partially replaced by the structural battery composite. The modelling results illustrated that a driving distance could increase by approximately 70% for BMW i3 with maintained original vehicle weight by introducing the structural battery design.
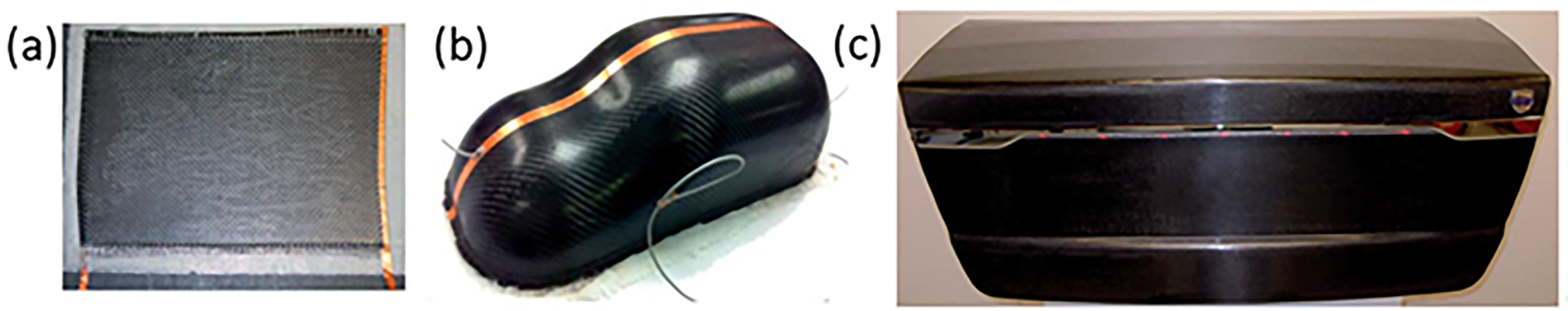
Figure 23. (a) Flat Laminate Structural Supercapacitor, (b) Model Car Body, and (c) Trunk Lid with Lit LED Lights for Volvo S80. Reprinted/adapted with permission from [101].

Figure 24. Modelling Schematic of an EV by Using an Original Steel Roof, an Updated Roof Made from Epoxy and Carbon Fibres and a Multifunctional Roof Made from the Structural Battery. Reprinted/adapted with permission from [102].
Different from monofunctional centralized energy storage which is independent of the vehicle’s body, the distributed embodied energy storage technologies could perform structural components and electrochemical energy storage tasks at the same time, holding significant potential in mass and volume savings for EVs. Moreover, such technologies offer more flexibility and convenience in the design and application of EVs. Despite great efforts that have been devoted to exploring structural power batteries and supercapacitors to date, there are still several existing challenges limiting their practical application. Further research for these embodied energy storage technologies is urgently needed in optimizing the manufacturing procedure to scale up and improve the energy density, specific power and cycle stability.
4. Conclusion
Renewable energy with appropriate energy storage technologies enables lightweight, low-carbon development for automotive applications. Although conventional fossil fuel still contributes to approximately 70% of electricity and power generation today, with great potential and fast-development, an extensive usage of renewable energy will be achieved by combining appropriate energy storage technologies in near future. This has triggered tremendous efforts to explore advanced energy storage technologies for automotive applications. As centralized energy storage, LIBs with high energy density and long cycle life have been growing popular in EVs. To further improve energy density and safety, solid-state Li-metal batteries are expected to be used in EVs in the future. The hydrogen fuel cell can be an alternative solution to battery EVs for long-distance transportation. On the other hand, the distributed energy storage realized by embodied energy storage technologies is considered a promising solution for the development of lightweight and efficient production in the automotive industry. In the future, the energy density and specific power of embodied energy storage still need to be improved for real application in EVs.
Author Contributions: Conceptualization, L.W.; Writing—original draft preparation, X.Y.; Y.J., H.L., A.R., M.K., D.C.; Writing—review and editing, D.J.P., L.W.; All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by the SmartForming Research Base at Imperial College London.
Data Availability Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Ritchie, H. Cars, planes, trains: where do CO2 emissions from transport come from? Available online: https://ourworldindata.org/co2-emissions-from-transport (Accessed on 12 September 2022).
- REN21. Renewables 2020 global status report. Available online: https://ren21.net/gsr-2020/ (Accessed on 29 September 2022).
- REN21. Renewables 2021 global status report. Available online: https://www.ren21.net/gsr-2021/ (Accessed on 29 September 2022).
- BraffW.A.; MuellerJ.M.; TrancikJ.E. Value of storage technologies for wind and solar energy. Nature Climate Change, 2016, 6(10): 964-969. DOI: https://doi.org/10.1038/nclimate3045
- RoperW. High demand for lithium-ion batteries. Available online: https://www.statista.com/chart/23808/lithium-ion-battery-demand/ (Accessed on 16 September 2022).
- IEA. Hydroelectricity. Available online: https://www.iea.org/reports/hydroelectricity (Accessed on 23 September 2022).
- Ember. Data explorer. Available online: https://ember-climate.org/data/data-explorer/ (Accessed on 15 September 2022).
- Ember. Global electricity review 2022. Available online: https://ember-climate.org/insights/research/global-electricity-review-2022/ (Accessed on 15 September 2022).
- KamranM.; FazalM.R. Renewable energy conversion systems. Pittsburgh: Academic Press, 2021.
- OMEXOM. Hydro. Available online: https://www.omexom.com/expertises/power/hydro/ (Accessed on 15 September 2022).
- International Hydropower Association. Facts about hydropower. Available online: https://www.hydropower.org/iha/discover-facts-about-hydropower (Accessed on 15 September 2022).
- SaarinenL.; NorrlundP.; LundinU. Field measurements and system identification of three frequency controlling hydropower plants. IEEE Transactions on Energy Conversion, 2015, 30(3): 1061-1068. DOI: https://doi.org/10.1109/TEC.2015.2425915
- Chaudhury, B. Hydropower. London: Imperial College London, 2022.
- BartleA. Hydropower potential and development activities. Energy Policy, 2002, 30(14): 1231-1239. DOI: https://doi.org/10.1016/S0301-4215(02)00084-8
- MussaM.; TekaH.; AyichoH. Environmental impacts of hydropower and alternative mitigation measures. Current Investigations in Agriculture and Current Research, 2018, 2(2): 184-186. DOI: https://doi.org/10.32474/CIACR.2018.02.000133
- OSTI.GOV. Hydropower value study: current status and future opportunities. Available online: https://www.osti.gov/servlets/purl/1764624 (Accessed on 15 September 2022). DOI: https://doi.org/10.5902/1983734865414
- IRENA. Majority of new renewables undercut cheapest fossil fuel on cost. Available online: https://www.irena.org/news/pressreleases/2021/Jun/Majority-of-New-Renewables-Undercut-Cheapest-Fossil-Fuel-on-Cost (Accessed on 15 September 2022).
- GWEC. Global wind report 2021. Brussels, Belgium: Global Wind Energy Council, 2021.
- RitchieH.; RoserM.; RosadoP. Energy. Available online: https://ourworldindata.org/energy (Accessed on 20 February 2022).
- KomusanacI.; BrindleyG.; FraileD.; et al. Wind energy in Europe: 2020 statistics and the outlook for 2021-2025. Brussels, Belgium: WindEurope, 2021.
- Center for Sustainable Systems, University of Michigan. Wind Energy Factsheet: CSS07-09. Ann Arbor: University of Michigan, 2021.
- EducationEnergy. Betz limit. Available online: https://energyeducation.ca/encyclopedia/Betz_limit (Accessed on 13 September 2022).
- RodrÍguezP.; TimbusA.; TeodorescuR.; et al. Reactive power control for improving wind turbine system behavior under grid faults. IEEE Transactions on Power Electronics, 2009, 24(7): 1798-1801. DOI: https://doi.org/10.1109/TPEL.2009.2014650
- MolinaM.G.; MercadoP.E. Modelling and control design of pitch-controlled variable speed wind turbines. Al-Bahadly, I. Wind Turbines. Rijeka: IntechOpen, 2011: 373-402.
- BuljanA. 162 offshore wind farms up and running worldwide, 26 more under construction. Available online: https://www.offshorewind.biz/2021/02/09/162-offshore-wind-farms-up-and-running-worldwide-26-more-under-construction/ (Accessed on 22 February 2022).
- TylerS.; PhilippB.; PatrickD. 2019 cost of wind energy review: NREL/TP-5000-78471. Golden, CO: National Renewable Energy Laboratory, 2020.
- IEA. Offshore wind outlook 2019. Paris: IEA, 2019.
- WiserR.; RandJ.; SeelJ.; et al. Expert elicitation survey predicts 37% to 49% declines in wind energy costs by 2050. Nature Energy, 2021, 6(5): 555-565. DOI: https://doi.org/10.1038/s41560-021-00810-z
- PossnerA.; CaldeiraK. Geophysical potential for wind energy over the open oceans. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(43): 11338-11343. DOI: https://doi.org/10.1073/pnas.1705710114
- GWEC. Floating offshore wind-A global opportunity. Available online: https://gwec.net/floating-offshore-wind-a-global-opportunity/ (Accessed on 29 September 2022).
- Harrabin, R. Boris Johnson: Wind farms could power every home by 2030. British Broadcasting Corporation. 2020. Available online: https://www.bbc.co.uk/news/uk-politics-54421489 (Accessed on 12 November 2021).
- Orsted. Hornsea projects. Available online: https://hornseaprojects.co.uk/ (Accessed on 23 November 2021).
- Scott, ChapterK. 1: Introduction to electrolysis, electrolysers and hydrogen production. Scott, K. Electrochemical methods for hydrogen production. Cambridge, UK: Royal Society of Chemistry, 2020: 1-27. DOI: https://doi.org/10.1039/9781788016049-00001
- ChapmanA.; ItaokaK.; HiroseK.; et al. A review of four case studies assessing the potential for hydrogen penetration of the future energy system. International Journal of Hydrogen Energy, 2019, 44(13): 6371-6382. DOI: https://doi.org/10.1016/j.ijhydene.2019.01.168
- SukhatmeS.P.; NayakJ.K. Solar energy. 4th ed. New York: Mc Graw Hill Education, 2017.
- EIA. Solar explained: photovoltaics and electricity. Available online: https://www.eia.gov/energyexplained/solar/photovoltaics-and-electricity.php (Accessed on 20 September 2022).
- SansomR. Challenges of decarbonising space and water heating for a low carbon future. London: Imperial College London, 2022.
- GreeningB.; AzapagicA. Domestic solar thermal water heating: a sustainable option for the UK?. Renewable Energy, 2014, 63: 23-36. DOI: https://doi.org/10.1016/j.renene.2013.07.048
- IRENA. Future of solar photovoltaic: deployment, investment, technology, grid integration and socio-economic aspects. Abu Dhabi: International Renewable Energy Agency, 2019.
- IRENA. Renewable power generation costs in 2020. Available online: https://www.irena.org/publications/2021/Jun/Renewable-Power-Costs-in-2020 (Accessed on 18 September 2022).
- IEA. Renewables 2021. Paris: IEA, 2021.
- REN21. Renewables 2022 Global Status report. Available online: https://www.unep.org/resources/report/renewables-2022-global-status-report (Accessed on 29 September 2022).
- BrunK.; AllisonT.; DennisR. Thermal, mechanical, and hybrid chemical energy storage systems. London, United Kingdom: Academic Press, 2021.
- IEA. Electric cars fend off supply challenges to more than double global sales. Available online: https://www.iea.org/commentaries/electric-cars-fend-off-supply-challenges-to-more-than-double-global-sales (Accessed on 21 September 2022).
- RennerM.; ParajuliB.; FerroukhiR.; et al. Measuring the socio-economic footprint of the energy transition: the role of supply chains: Analysis built on renewable energy benefits: measuring the economics (IRENA, 2016). Available online: https://doi.org/10.13140/RG.2.2.12085.70887 (Accessed on 29 September 2022).
- IrleR.; EV-Volumes. Global EV sales for 2022 H1. Available online: https://www.ev-volumes.com/ (Accessed on 22 September 2022).
- Bullock, Lead/acid batteriesK.R.. Journal of Power Sources, 1994, 51(1/2): 1-17. DOI: https://doi.org/10.1016/0378-7753(94)01952-5
- WeinertJ.X.; BurkeA.F.; WeiX.Z. Lead-acid and lithium-ion batteries for the Chinese electric bike market and implications on future technology advancement. Journal of Power Sources, 2007, 172(2): 938-945. DOI: https://doi.org/10.1016/j.jpowsour.2007.05.044
- XiaW.; MahmoodA.; ZouR.Q.; et al. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy & Environmental Science, 2015, 8(7): 1837-1866. DOI: https://doi.org/10.1039/C5EE00762C
- JungJ.; ZhangL.; ZhangJ.J. Lead-acid battery technologies. Boca Raton: CRC Press, 2015.
- HugginsR. Energy storage: fundamentals, materials and applications. 2nd ed. Cham: Springer International Publishing, 2016. DOI: https://doi.org/10.1007/978-3-319-21239-5
- LiuY.F.; PanH.G.; GaoM.X.; et al. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries. Journal of Materials Chemistry, 2011, 21(13): 4743-4755. DOI: https://doi.org/10.1039/C0JM01921F
- CanoZ.P.; BanhamD.; YeS.Y.; et al. Batteries and fuel cells for emerging electric vehicle markets. Nature Energy, 2018, 3(4): 279-289. DOI: https://doi.org/10.1038/s41560-018-0108-1
- FetcenkoM.A.; OvshinskyS.R.; ReichmanB.; et al. Recent advances in NiMH battery technology. Journal of Power Sources, 2007, 165(2): 544-551. DOI: https://doi.org/10.1016/j.jpowsour.2006.10.036
- OuyangL.Z.; HuangJ.L.; WangH.; et al. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: a review. Materials Chemistry and Physics, 2017, 200: 164-178. DOI: https://doi.org/10.1016/j.matchemphys.2017.07.002
- ZhanF.; JiangL.J.; WuB.R.; et al. Characteristics of Ni/MH power batteries and its application to electric vehicles. Journal of Alloys and Compounds, 1999, 293/295: 804-808. DOI: https://doi.org/10.1016/S0925-8388(99)00361-8
- ArmandM.; TarasconJ.M. Building better batteries. Nature, 2008, 451(7179): 652-657. DOI: https://doi.org/10.1038/451652a
- LarcherD.; TarasconJ.M. Towards greener and more sustainable batteries for electrical energy storage. Nature Chemistry, 2015, 7(1): 19-29. DOI: https://doi.org/10.1038/nchem.2085
- ChenZ.H.; LeeD.J.; SunY.K.; et al. Advanced cathode materials for lithium-ion batteries. MRS Bulletin, 2011, 36(7): 498-505. DOI: https://doi.org/10.1557/mrs.2011.155
- WhittinghamM.S. Lithium batteries and cathode materials. Chemical Reviews, 2004, 104(10): 4271-4301. DOI: https://doi.org/10.1021/cr020731c
- AndrewsA.; SinghA.; SenguptaS. Structural battery. TechScape: The Science, Technology and Education Journal of IIT Jodhpur. 2022.
- PejmanR.; KumburE.C.; NajafiA.R. Multi-physics design optimization of structural battery. Multifunctional Materials, 2021, 4(2): 024001. DOI: https://doi.org/10.1088/2399-7532/abf158
- SchmuchR.; WagnerR.; HörpelG.; alet, Performance and cost of materials for lithium-based rechargeable automotive batteries. Nature Energy, 2018, 3(4): 267-278. DOI: https://doi.org/10.1038/s41560-018-0107-2
- SawL.H.; YeY.H.; TayA.A.O. Integration issues of lithium-ion battery into electric vehicles battery pack. Journal of Cleaner Production, 2015, 113: 1032-1045. DOI: https://doi.org/10.1016/j.jclepro.2015.11.011
- KwadeA.; HaselriederW.; LeithoffR.; et al. Current status and challenges for automotive battery production technologies. Nature Energy, 2018, 3(4): 290-300. DOI: https://doi.org/10.1038/s41560-018-0130-3
- SaktiA.; MichalekJ.J.; FuchsE.R.H.; et al. A techno-economic analysis and optimization of Li-ion batteries for light-duty passenger vehicle electrification. Journal of Power Sources, 2015, 273: 966-980. DOI: https://doi.org/10.1016/j.jpowsour.2014.09.078
- MaiserE. Battery packaging-technology review. AIP Conference Proceedings, 2014, 1597(1): 204. DOI: https://doi.org/10.1063/1.4878489
- XuW.; WangJ.L.; DingF.; et al. Lithium metal anodes for rechargeable batteries. Energy & Environmental Science, 2014, 7(2): 513-537. DOI: https://doi.org/10.1039/C3EE40795K
- LiW.; DahnJ.R.; WainwrightD.S. Rechargeable lithium batteries with aqueous electrolytes. Science, 1994, 264(5162): 1115-1118. DOI: https://doi.org/10.1126/science.264.5162.1115
- TarasconJ.M.; ArmandM. Issues and challenges facing rechargeable lithium batteries. Nature, 2001, 414(6861): 359-367. DOI: https://doi.org/10.1038/35104644
- LiJ.C.; MaC.; ChiM.F.; et al. Solid electrolyte: the key for high-voltage lithium batteries. Advanced Energy Materials, 2015, 5(4): 1401408. DOI: https://doi.org/10.1002/aenm.201401408
- GoodenoughJ.B.; HongH.Y.P.; Kafalas J.A.; et al. Fast Na+-ion transport in skeleton structures. Materials Research Bulletin, 1976, 11(2): 203-220. DOI: https://doi.org/10.1016/0025-5408(76)90077-5
- JackmanS.D.; CutlerR.A. Stability of NaSICON-type Li1.3Al0.3Ti1.7P3O12 in aqueous solutions. Journal of Power Sources, 2013, 230: 251-260. DOI: https://doi.org/10.1016/j.jpowsour.2012.12.022
- LiY.T.; HanJ.T.; WangC.A.; et al. Optimizing Li+ conductivity in a garnet framework. Journal of Materials Chemistry, 2012, 22(30): 15357-15361. DOI: https://doi.org/10.1039/c2jm31413d
- ZhuJ.X.; LiX.L.; WuC.W.; et al. A multilayer ceramic electrolyte for all-solid-state Li batteries. Angewandte Chemie International Edition, 2021, 60(7): 3781-3790. DOI: https://doi.org/10.1002/anie.202014265
- InagumaY.; ChenL.Q.; Itoh, M; et al. High ionic conductivity in lithium lanthanum titanate. Solid State Communications, 1993, 86(10): 689-693. DOI: https://doi.org/10.1016/0038-1098(93)90841-A
- YuR.; DuQ.X.; ZouB.K.; et al. Synthesis and characterization of perovskite-type (Li,Sr)(Zr,Nb)O3 quaternary solid electrolyte for all-solid-state batteries. Journal of Power Sources, 2016, 306: 623-629. DOI: https://doi.org/10.1016/j.jpowsour.2015.12.065
- ShiZ.Q.; GuoW.Y.; ZhouL.Z.; et al. A 3D fiber skeleton reinforced PEO-based polymer electrolyte for high rate and ultra-long cycle all-solid-state batteries. Journal of Materials Chemistry A, 2021, 9(37): 21057-21070. DOI: https://doi.org/10.1039/D1TA04619E
- ZhangX.; LiuT.; ZhangS.F.; et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12and poly (vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. Journal of the American Chemical Society, 2017, 139(39): 13779-13785. DOI: https://doi.org/10.1021/jacs.7b06364
- FangR.Y.; XuB.Y.; GrundishN.S.; et al. Li2S6-integrated PEO-based polymer electrolytes for all-solid-state lithium-metal batteries. Angewandte Chemie International Edition, 2021, 60(32): 17701-17706. DOI: https://doi.org/10.1002/anie.202106039
- YangK.; ChenL.K.; MaJ.B.; et al. Stable interface chemistry and multiple ion transport of composite electrolyte contribute to ultra-long cycling solid-state LiNi0.8 Co0.1 Mn0.1 O2/lithium metal batteries. Angewandte Chemie International Edition, 2021, 60(46): 24668-24675. DOI: https://doi.org/10.1002/anie.202110917
- IEA. Technology roadmap - hydrogen and fuel cells. Paris: IEA, 2015.
- ZhaoG.L.; NielsenE.R.; TroncosoE.; et al. Life cycle cost analysis: a case study of hydrogen energy application on the Orkney Islands. International Journal of Hydrogen Energy, 2019, 44(19): 9517-9528. DOI: https://doi.org/10.1016/j.ijhydene.2018.08.015
- WeiM.; SmithS.J.; SohnW.D. Experience curve development and cost reduction disaggregation for fuel cell markets in Japan and the US. Applied Energy, 2017, 191: 346-357. DOI: https://doi.org/10.1016/j.apenergy.2017.01.056
- MiottiM.; HoferJ.; BauerC. Integrated environmental and economic assessment of current and future fuel cell vehicles. The International Journal of Life Cycle Assessment, 2017, 22(1): 94-110. DOI: https://doi.org/10.1007/s11367-015-0986-4
- AlazemiJ.; AndrewsJ. Automotive hydrogen fuelling stations: an international review. Renewable and Sustainable Energy Reviews, 2015, 48: 483-499. DOI: https://doi.org/10.1016/j.rser.2015.03.085
- AubinC.A.; GorissenB.; MilanaE.; et al. Towards enduring autonomous robots via embodied energy. Nature, 2022, 602(7897): 393-402. DOI: https://doi.org/10.1038/s41586-021-04138-2
- AspL.E.; JohanssonM.; LindberghG.; et al. Structural battery composites: a review. Functional Composites and Structures, 2019, 1(4): 042001. DOI: https://doi.org/10.1088/2631-6331/ab5571
- PereiraT.; GuoZ.H.; NiehS.; et al. Embedding thin-film lithium energy cells in structural composites. Composites Science and Technology, 2008, 68(7/8): 1935-1941. DOI: https://doi.org/10.1016/j.compscitech.2008.02.019
- RobertsS.C.; AgliettiG.S. Structural performance of a multifunctional spacecraft structure based on plastic lithium-ion batteries. Acta Astronautica, 2010, 67(3/4): 424–439. DOI: https://doi.org/10.1016/j.actaastro.2010.03.004
- WangY.; PengC.Y.; ZhangW.H.; et al. Mechanical and electrical behavior of a novel satellite multifunctional structural battery. Journal of Scientific and Industrial Research, 2014, 73(3): 163-167.
- LadpliP.; NardariR.; KopsaftopoulosF.; et al. Multifunctional energy storage composite structures with embedded lithium-ion batteries. Journal of Power Sources, 2019, 414: 517-529. DOI: https://doi.org/10.1016/j.jpowsour.2018.12.051
- ZhangY.C.; MaJ.; SinghA.K.; et al. Multifunctional structural lithium-ion battery for electric vehicles. Journal of Intelligent Material Systems and Structures, 2017, 28(12): 1603-1613. DOI: https://doi.org/10.1177/1045389X16679021
- AspL.E.; BoutonK.; CarlstedtD.; et al. A structural battery and its multifunctional performance. Advanced Energy and Sustainability Research, 2021, 2(3): 2000093. DOI: https://doi.org/10.1002/aesr.202000093
- AspL.E.; GreenhalghE.S. Structural power composites. Composites Science and Technology, 2014, 101: 41-61. DOI: https://doi.org/10.1016/j.compscitech.2014.06.020
- LendleinA.; TraskR.S. Multifunctional materials: concepts, function-structure relationships, knowledge-based design, translational materials research. Multifunctional Materials, 2018, 1(1): 010201. DOI: https://doi.org/10.1088/2399-7532/aada7b
- LiuP.; ShermanE.; JacobsenA. Design and fabrication of multifunctional structural batteries. Journal of Power Sources, 2009, 189(1): 646-650. DOI: https://doi.org/10.1016/j.jpowsour.2008.09.082
- WongE.L.; BaechleD.M.; XuK.; et al. Design and processing of structural composite batteries. Proceedings of Society for the Advancement of Materiel and Process Engineering (SAMPE) 2007 Symposium and Exhibition. Baltimore, Maryland: SAMPE, 2007: 1-16.
- EkstedtS.; WysockiM.; AspL.E. Structural batteries made from fibre reinforced composites. Plastics, Rubber and Composites, 2010, 39(3/5): 148-150. DOI: https://doi.org/10.1179/174328910X12647080902259
- MoyerK.; BoucherbilN.A.; ZohairM.; et al. Polymer reinforced carbon fiber interfaces for high energy density structural lithium-ion batteries. Sustainable Energy & Fuels, 2020, 4(6): 2661-2668. DOI: https://doi.org/10.1039/D0SE00263A
- ShirshovaN.; QianH.; HoulléM.; et al. Multifunctional structural energy storage composite supercapacitors. Faraday Discussions, 2014, 172: 81-103. DOI: https://doi.org/10.1039/C4FD00055B
- JohannissonW.; ZenkertD.; LindberghG.; et al. Model of a structural battery and its potential for system level mass savings. Multifunctional Materials, 2019, 2(3): 035002. DOI: https://doi.org/10.1088/2399-7532/ab3bdd
- CarlstedtD.; AspL.E. Performance analysis framework for structural battery composites in electric vehicles. Composites Part B: Engineering, 2020, 186: 107822. DOI: https://doi.org/10.1016/j.compositesb.2020.107822






