Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
Exhaust Gas After-Treatment Systems for Gasoline and Diesel Vehicles
Liye Bao 1, Jihua Wang 2, Lihui Shi 2, and Haijun Chen 1, *
1 College of Electronic Information and Optical Engineering, Nankai University, Tianjin 300350, China
2 Valiant Co., Ltd., Yantai 264006, China
* Correspondence: chenhj@nankai.edu.cn
Received: 5 October 2022
Accepted: 10 November 2022
Published: 25 December 2022
Abstract: Exhaust gases released from vehicle engines have been a major cause of air pollution, and the emission limits have become much stricter in recent years due to a worldwide concern about the impact of air pollution on public health. These regulations have been complied to minimize the emissions of carbon monoxide (CO), hydrocarbons (HCs), nitrogen oxides (NO x) and particulate matter (PM) from gasoline and diesel vehicles engines. Different after-treatment systems (ATS) have been developed for the treatment of exhaust gases from gasoline and diesel engines, respectively. The ATS for gasoline engine based on the three-way catalysts (TWCs), as well as the ATS for diesel engines including diesel oxidation catalysts (DOC), selective catalytic reduction (SCR), diesel particulate filters (DPF) and ammonia slip catalysts (ASC), are summarized in this mini-review.
Keywords:
after-treatment systems (ATS) three-way catalysts (TWCs) catalysts zeolite diesel oxidation catalysts (DOC) selective catalytic reduction (SCR) diesel particulate filters (DPF) ammonia slip catalysts (ASC)1. Introduction
The health risks of air pollution are extremely concerned around the world now, especially for the harmful air pollutants which are emitted from vehicles. Over the past four decades, several sets of emission standards have been defined for different types of vehicles. As shown in Table 1, the emission limits have become stricter and stricter both in both developed and developing countries [ 1]. Different after-treatment system (ATS) have been successfully developed for the treatment of exhaust gases from vehicles to meet these emission regulations, of which environment catalysis technologies have play important roles [ 2].
Table 1. Emission standards for diesel engines of heavy-duty vehicles (g kW -1 h -1) and gasoline of light-duty vehicles (g km -1) 1.

The classic gasoline engine ATS has built itself well on the three-way catalysts (TWCs) for over 30 years, which convert hazardous carbon monoxide (CO), nitrogen oxides (NO x), unburnt hydrocarbons (HC) into harmless products carbon dioxide (CO 2), nitrogen (N 2) and water (H 2O) [ 3]. Unfortunately, the TWCs catalytic technology does not work well for the control of NO x emissions on lean-burn engine operations with high oxygen/fuel ratios [ 4]. Therefore, selective catalytic reduction (SCR) of NO x into N 2 with the reductant such as ammonia, has been commercialized for the abatement of NO x from diesel engine vehicles [ 5].
2. The After-Treatment Systems for Gasoline Vehicles
2.1. The Origin of Three-Way Catalysts (TWCs)
The first generation of automobile catalyst was developed by Engerlhard (acquired by BASF in 2006) in the 1970s. The main active ingredients were platinum (Pt) and palladium (Pd) which were used to eliminate CO and HC. NO x was reduced by a mechanical system called exhaust gas recirculation (EGR). However, because of the widespread addition of lead (Pb) additives in gasoline that was adopted to improve the octane number of gasoline, the catalyst had been plagued by Pb poisoning. Fortunately, a legislation was called for the elimination of Pb additives due to environmental concerns and mounting evidence of its toxicity.
With the implementation of NO x regulations in 1980s, it was found that EGR systems cannot meet the requirement of 90% conversion of NO x. Research on catalytic materials led to the discovery of Rhodium (Rh) as a highly active and stable catalyst for the reduction of NO/NO 2. [ 6] Pt and Pd were the best solution for the oxidation reactions of both CO and HC, while Pt/Pd/Rh became the main active components of the TWCs system. However, Farrauto et al. [7] reported different air-to-fuel feed ratios can affect the performance of TWCs. When there is fuel rich or deficient in O 2, it will facilitate the reduction of NO x to N 2. On the other hand, when there is fuel lean or excess air, the oxidation reactions will be favourable. In order to control the engine operation and provide the narrow air-to-fuel ratio (λ = 1) , the first automotive Lambda sensor, also known as an oxygen sensor, was developed by Bosch in 1976 to monitor the amount of unburnt oxygen present in the exhaust pipe. Since then, TWCs exhibited their great advantage for the reduction of NO x as well as the oxidation of CO and HC simultaneously.
2.2. The Composition of TWCs
Currently, about 98% of the cars with gasoline engines have been equipped with TWCs. As shown in Figure 1, Bahaloo-Horeh [8] described that a TWCs catalytic converter mainly consists of cordierites or metal base integral substrates with honeycomb structures, which are coated with high surface area oxide supports, catalytic active metals, as well as oxygen storage materials as the promoters.
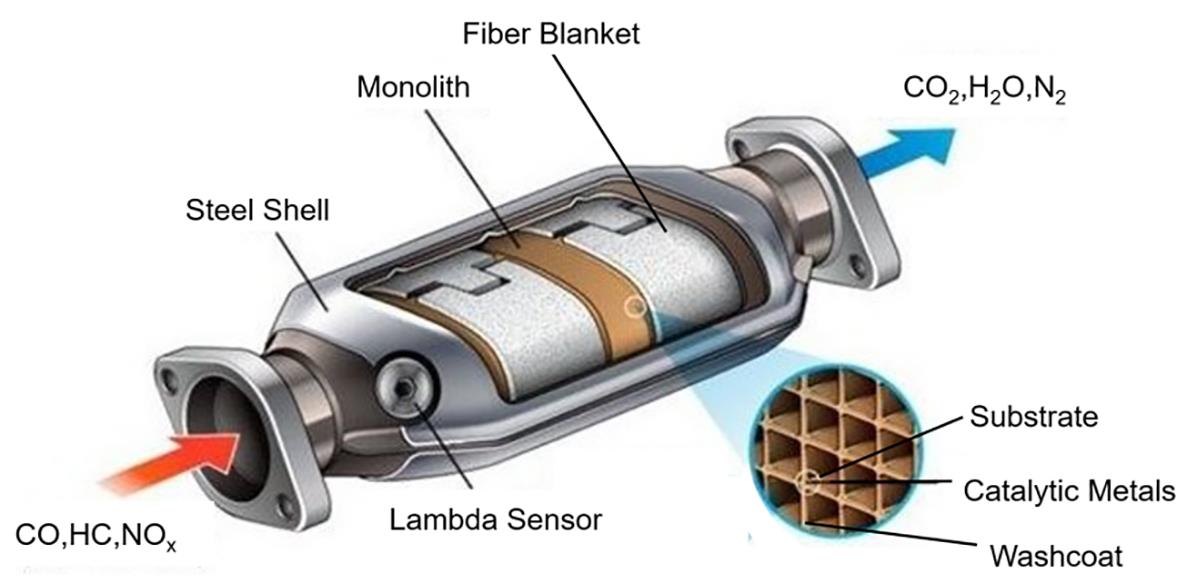
Figure 1. The composition of after-treatment systems (ATS) for gasoline vehicles.
Two kinds of monolith for TWCs have been used with cordierite (2MgO·2Al 2O 3·5SiO 2) and metal, respectively [ 9]. Cordierite monoliths have been widely used due to their advantages of low costs, good thermal shock resistance, remarkably low thermal expansion coefficients and high melting temperatures [ 10]. Such advantages make cordierites a good material that can withstand the harsh conditions of TWCs. On the other hand, a major advantage of metallic monoliths is their low heat capacity and high thermal conductivity, which allows the close-coupled catalyst (CCC) to be heated fast during the phase-in of the engine, and results in the minimization of the light-off time [ 11].
Platinum (Pt), palladium (Pd), and rhodium (Rh) are the common clusters of platinum-group metals (PGMs), which are dispersed onto the surface of oxide supports and employed as the active components in TWCs designs [ 12]. Pt and Pd are used as oxidizing components for three-way catalysis, while Rh is necessary for the control of NO x emissions. Currently, Pd-only, Pd/Rh, Pd/Pt/Rh, and Pt/Rh have been all commercialized for TWCs formulations [ 13]. Alumina (Al 2O 3) is a primary material in most TWCs washcoat formulations due to its high surface area, mechanical strength and good thermal stability [ 14]. In most of the cases, γ-Al 2O 3 is used as the first coating layer on the monolithic cordierite as a support of noble metal catalysts. Alkaline, alkaline earth metals and lanthanides are usually combined with Al 2O 3 as structural stabilizers to inhibit the conversion of Al 2O 3 from γ to α phase [ 15]. The introduction of CeO 2 as an Oxygen Storage Capacitor (OSC) enables a constant air-fuel ratio around the stoichiometry resulting in an enhancement on the catalytic activity and the durability for Pd-based catalysts [ 16]. Barium (Ba) has been reported to play an important role for the inhibition of the aggregation Pd active sites, which is indispensable for high performance catalysts [ 17]. Jing et al. [18] reported that Pd/La/Al 2O 3 can promote the catalytic reduction of NO efficiently, while Pd/Ba/Al 2O 3 exhibits high activity for the oxidation of CO and C 3H 6. In addition, TWCs formulations are often influenced by economic factors, regulatory standards, fuel quality and different types of exhaust composition.
2.3. Prospects of TWCs
As discussed above, TWCs have shown a quite mature and highly effective performance for pollution abatement. However, there are some inherent limitations which require further improvement and development. One of the biggest issues for TWCs is the degradation of the catalytic activity caused by the agglomerating of noble metals. Understanding the influence of the temperature and atmosphere (on the state of noble metals and the noble metals-carrier interaction) is the key to realize the stabilization of noble metal active sites. For example, Chen et al. [19] reported that the composition of aging gases impacted the particle size of Pd. Aging treatment under oxygen-rich conditions with the co-existence of steams at a high temperature led to much more serious agglomerating of noble metals. In contrast, particle growth is minimal in a mixture of depleted (O 2) gases below the decomposition temperature of PdO, and the sintered Pd particles could be modestly re-dispersed by treating in O 2. Therefore, it is very important to develop advanced TWCs synthesis methods and engine control technologies, which undoubtedly plays a critical role for the reduction of the usage of noble metals and the cost of TWCs.
The low activity at low temperatures (start-up of the engine) is another challenge for TWCs systems. The majority of the tailpipe emissions are emitted under low temperature operating conditions of the vehicle, especially during the cold start periods. The first possibility technology to reduce the cold start emissions is HCs traps, and typically HCs trap materials consist of zeolites and TWCs component. In an optimal trap, HCs emissions are trapped on zeolites at low temperatures. As the temperature is increased to above 250–300 °C, the stored HCs are released from zeolite components and subsequently converted on TWCs component. However, a major technical hurdle is that the temperature of HCs release is still relatively low comparing with the light-off temperature of TWCs. Moreover, the temperature of the under-floor TWCs catalysts may reach 850–900 ℃. Thus, a high stability under hydrothermal conditions is necessary for HCs trap materials w, but remains a challenge for zeolite-based systems currently [ 20]. As a result, the HCs trap technology has been employed only in some limited commercial applications so far. In addition, another approach is to develop new catalysts with ultra-high conversion efficiency at a low temperature in order to meet the stringent emission regulations in the future.
3. The After-Treatment Systems for Diesel Vehicles
3.1. Principles of the Operation
Diesel vehicles emissions are significantly dependent on the performance of the ATS. As shown in Figure 2, Beale et al. [21] described that a typical ATS is composed of diesel oxidation catalysts (DOC), selective catalytic reduction (SCR), diesel particulate filters (DPF) and ammonia slip catalysts (ASC) coated on honeycomb substrates such as cordierite, which is now used to meet Euro 6.

Figure 2. Layout of ATS for diesel vehicles.
TWCs is an effective NO x reduction system without the presence of oxygen while it does not work well for the control of NO x emissions on lean-burn engine operatiosn with high oxygen/fuel ratios. Thus, selective catalytic reduction of NO x into N 2 (where ammonia is used as the reductant) has been commercialized for the deNO x treatment of diesel vehicles [ 22]. The main reactions on SCR catalysts are shown as follows:
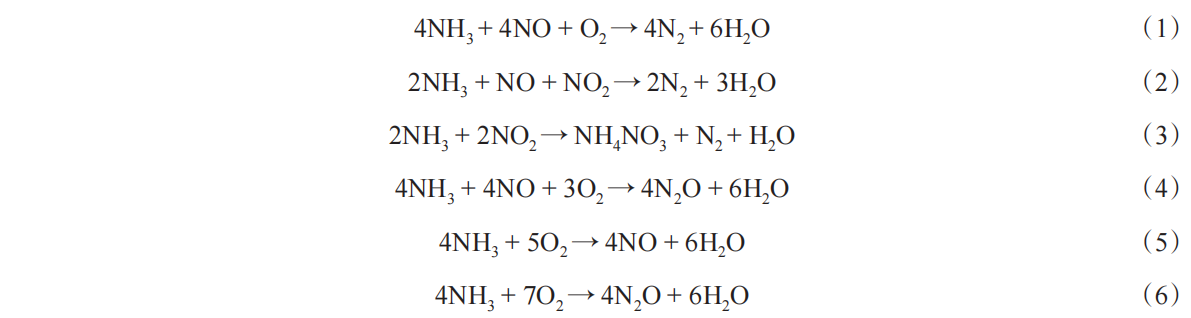
As NO x in diesel exhaust usually consists of >90% NO, the main reaction of SCR with NH 3 is the standard SCR reaction (SSCR) (1). When NO and NO 2 are ideally in equimolar amounts, especially at low temperatures, the conversion of NO x by the reaction with NH 3 proceeds through the fast SCR reaction (FSCR) (2) [ 23]. In addition to the standard and fast SCR reactions, there are several side reactions occurring simultaneously. In the low-temperature regions, NH 4NO 3 will be accumulated through reaction (3). In the high-temperature regions, the non-SCR and catalytic oxidation of NH 3 will occur through reactions (4-6). Therefore, good NH 3-SCR catalysts and their systems should be developed to promote the reactions of (1) and (2) and inhibit the reactions of (4)~(6) [ 24].
3.2. Diesel Oxidation Catalyst (DOC)
The DOC unit is typically the first component in the Diesel ATS with platinum-group metal (PGM) as catalytic active species. The DOC unit serves in commercial emission control systems with two major functions. First, it converts hydrocarbon (HCs) and CO into CO 2 and water. Second, NO can be oxidized to NO 2 on DOC, while a higher proportion of NO 2 is beneficial to improve the NO x conversion efficiency of SCR [ 25]. Moreover, it can create exothermic heat which can be used to regenerate DPF due to the exothermic oxidation reaction on DOC units.
3.3. Selective Catalytic Reduction (SCR)
Selective catalytic reduction with ammonia (NH 3-SCR) is a technology used to abate nitrogen oxide emissions from diesel engines effectively [ 26]. Vanadium-based oxides, copper zeolite, and iron zeolite are three general families of SCR catalysts for commercial use today [ 27]. Copper zeolite has been the leading choice due to the high activity under a wide temperature window, among which small-pore zeolites such as CHA have shown especially good hydrothermal stability and excellent resistance to HC poisoning [ 28]. As a result, Cu/SSZ-13 zeolite has been employed for the high-performing SCR systems as the most popular SCR catalyst [ 29]. Comparing with copper zeolite, iron zeolite exhibits a better catalytic performance at a high temperature, as well as a lower affinity for sulphur. However, the inferior low-temperature activity of iron zeolite has hampered its applications to meet stringent emission limits with a low exhaust temperature [ 30]. Vanadium-based oxides catalysts are generally preferred for diesel engine using high-sulphur fuels due to their greater sulfur resistance. However, the key drawback of these catalysts is their poor stability at a high temperature [ 31].
3.4. Diesel Particulate Filters (DPF)
The combustion patterns of diesel engines usually result in high emissions of particulate matter (PM). Therefore, DPF have become a necessary configuration to meet increasingly stringent PM emission standards [ 32]. Modern DPF is a wall-flow ceramic monolithic filter, which mainly consists of cordierite, silicon carbide or aluminum titanate. Engine exhaust gas is forced to flow through a porous wall to adjacent outlet channels of the DPF, then most soot agglomerates accumulate on the filter [ 33]. To keep the filter clean, the collected soot must be burnt via DPF regeneration.
3.5. Ammonia Slip Catalyst (ASC)
Generally, excess ammonia is injected to ensure the high conversion efficiency of NH 3-SCR deNO x systems. However, some unreacted ammonia may slip through the SCR units. ASC catalysts are normally coated on the honeycomb substrate with PGM catalyst, which can oxidize slipped ammonia to N 2 [ 34]. Recent developments in ASC catalysts technology is a dual-layer structure in which the PGM catalyst coating is located below the SCR catalyst coating. The bottom PGM layer oxidizes NH 3 to N 2 with the byproduct of NO x, while these latter compounds diffuse back to the above layer in which they are also transformed into N 2 with the excess NH 3 typically stored by the SCR catalyst. Thus, this solution shows great advantages in terms of NH 3 overall conversion and N 2 selectivity [ 35, 36].
3.6. Prospects of After-Treatment Systems (ATS) for Diesel Vehicles
Despite SCR is the leading lean deNO x technology today, there are still plenty of opportunities for the improvement on SCR systems for the ATS of diesel vehicles. One significant opportunity is to improve the catalytic performance at low temperatures below 200 ℃. The cold-start deNO x performance remains a great challenge considering the kinetic limitations of SCR catalysts and the decomposition of urea at low temperatures. PNNL, Cummins Inc. and Johnson-Matthey Inc. have collaborated to develop a sustained low-temperature NO x reduction (SLTNR) system capable of removing 90% of NO x at SCR inlet temperatures of 150 °C. The key of this new technology is the utilization of fast SCR instead of standard SCR so as to boost NO x conversion. As a result, a diesel oxidation catalyst (DOC) bed is required to oxidize NO to NO 2. [ 37] A second solution to solve the cold-start problem is the usage of passive NO x adsorbers technology (PNAs), of which the adsorbent materials are invented to store all NO x emitted after the engine start-up until the exhaust temperature is high enough for the SCR reaction. For example, the group of Tronconi showed a new two-layer monolithic AdSCR system (AdSCR=adsorption + selective catalytic reduction), which was constituted by Cu/CHA (top layer) and BaO/Al 2O 3 (bottom layer), to improve the deNO x performances of ATS for diesel vehicles at low temperatures [ 38]. Another opportunity is to further improve the stability of SCR systems. Especially, the hydrothermal stability of Cu/SSZ-13 zeolite can be considered as a critical criterion for the evaluation of the long-term stability for practical applications [ 39]. As the dealumination and the loss of surface area/pore volume of zeolites, which lead to the transformation of Cu active species into inactive forms, do occur during the hydrothermal aging, new zeolites should be designed and synthesized to withstand harsh hydrothermal aging tests.
4. Concluding Remarks
In this review, a number of after-treatment devices have been summarized. It can be found that no single setup is sufficient to eliminate exhaust pollutant completely. New catalytic materials and control technologies remain as impending demand to meet much stricter limits and rules such as Euro 7. Scientific understanding on the catalytic mechanism, rational design of materials and ATS, as well as precise powder coating should be further investigated and developed not only for ATS of gasoline vehicles but also for that of diesel vehicles with high decontaminating efficiency. Besides, ATS of automobiles should also have compact design with low costs to benefit both procurements and operations.
It has been well acknowledged that anthropogenic emissions of CO 2 are the main cause to the continuous increase of the global temperature since the industrial revolution. Many CO 2 mitigation strategies have been under investigation in the past years with the goal of net-zero emissions by 2050 [ 40]. New engine systems and their exhaust gas after-treatment systems should be developed with the usage of zero-carbon fuels and biofuels such as ammonia, biogas and synthetic hydrocarbon with carbon capture.
Author Contributions: Investigation, writing—original draft preparation, L.B.; investigation, J.W.; investigation, L.S.; writing—review, editing, funding acquisition, H. C. All authors have read and agreed to the published version of the manuscript.
Funding: The work was supported by Chinese National Natural and Science Foundation 22076081.
Data Availability Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Huang C. ; Shan W. ; Lian Z. ; et al . Recent advances in three-way catalysts of natural gas vehicles. Catal. Sci. Technol. 2020, 10( 19), 6407– 6419. DOI: https://doi.org/10.1039/D0CY01320J
- Lloyd A.C. ; Cackette T . A. Diesel engines: environmental impact and control. J. Air Waste Manag. Assoc. 2001, 51( 6), 809‒ 47. DOI: https://doi.org/10.1080/10473289.2001.10464315
- Zhang N. ; Ye C. ; Yan H. ; et al . Single-atom site catalysts for environmental catalysis. Nano Res. 2020, 13( 12), 3165‒ 3182. DOI: https://doi.org/10.1007/s12274-020-2994-3
- Dusselier, M.; Davis, M. E. Small-Pore Zeolites: Synthesis and Catalysis. Chem. Rev. 2018, 118( 11), 5265‒ 5329. DOI: https://doi.org/10.1021/acs.chemrev.7b00738
- Han L. ; Cai S. ; Gao M. ; et al . Selective Catalytic Reduction of NOx with NH 3 by Using Novel Catalysts: State of the Art and Future Prospects . Chem. Rev. 2019, 119( 19), 10916‒ 10976. DOI: https://doi.org/10.1021/acs.chemrev.9b00202
- Hu Z. ; Allen F.M. ; Wan C.Z. ; et al . Performance and structure of Pt–Rh three-way catalysts: mechanism for Pt/Rh synergism. J. Catal. 1998, 174, 13‒ 21. DOI: https://doi.org/10.1006/jcat.1997.1954
- Farrauto R.J. ; Deeba M. ; Alerasool S . Gasoline automobile catalysis and its historical journey to cleaner air. Nat. Catal. 2019, 2( 7), 603‒ 613. DOI: https://doi.org/10.1038/s41929-019-0312-9
- Bahaloo-Horeh N. ; Mousavi S . M. Comprehensive characterization and environmental risk assessment of end-of-life automotive catalytic converters to arrange a sustainable roadmap for future recycling practices. J. Hazard. Mater. 2020, 400, 123186. DOI: https://doi.org/10.1016/j.jhazmat.2020.123186
- Santos H. ; Costa M . Evaluation of the conversion efficiency of ceramic and metallic three way catalytic converters. Energy Convers. Manag. 2008, 49( 2), 291‒ 300. DOI: https://doi.org/10.1016/j.enconman.2007.06.008
- Rood S. ; Eslava S. ; Manigrasso A. ; et al . Recent advances in gasoline three-way catalyst formulation: A review. Proc. Inst. Mech. Eng., Part D 2019, 234( 4), 936‒ 949. DOI: https://doi.org/10.1177/0954407019859822
- Kašpar J. ; Fornasiero P. ; Hickey N . Automotive catalytic converters: current status and some perspectives. Catal. Today 2003, 77( 4), 419‒ 449. DOI: https://doi.org/10.1016/S0920-5861(02)00384-X
- Koga H. ; Hayashi A. ; Ato Y. ; et al . Effect of ceria and zirconia supports on NO reduction over platinum-group metal catalysts: A DFT study with comparative experiments. Catal. Today 2019, 332, 236‒ 244. DOI: https://doi.org/10.1016/j.cattod.2018.07.023
- Wang J. ; Chen H. ; Hu Z. ; et al . A Review on the Pd-Based Three-Way Catalyst. Catal. Rev. 2014, 57( 1), 79‒ 144. DOI: https://doi.org/10.1080/01614940.2014.977059
- Machida M. ; Uchida Y. ; Ishikawa Y. ; et al . Thermostable Rh Metal Nanoparticles Formed on Al2O3 by High-Temperature H2 Reduction and Its Impact on Three-Way Catalysis. J. Phys. Chem. C 2019, 123( 40), 24584‒ 24591. DOI: https://doi.org/10.1021/acs.jpcc.9b06657
- Vedyagin A.A. ; Kenzhin R.M. ; Tashlanov M.Y. ; et al . Effect of La Addition on the Performance of Three-Way Catalysts Containing Palladium and Rhodium. Top. Catal. 2020, 63( 1), 152‒ 165. DOI: https://doi.org/10.1007/s11244-019-01213-x
- Li L. ; Zhang N. ; Wu R. ; et al . Comparative Study of Moisture-Treated Pd@CeO2/Al2O3 and Pd/CeO2/Al2O3 Catalysts for Automobile Exhaust Emission Reactions: Effect of Core-Shell Interface. ACS Appl. Mater. Interfaces 2020, 12( 9), 10350‒ 10358. DOI: https://doi.org/10.1021/acsami.9b20734
- Jing Y. ; Wang G. ; Mine S. ; et al . Role of Ba in an Al2O 3-Supported Pd-based Catalyst under Practical Three-Way Catalysis Conditions . ChemCatChem 2022, 14( 8), e202101462. DOI: https://doi.org/10.1002/cctc.202101462
- Jing Y. ; Wang G. ; Ting K.W. ; et al . Roles of the basic metals La, Ba, and Sr as additives in Al2O3-supported Pd-based three-way catalysts. J. Catal. 2021, 400, 387‒ 396. DOI: https://doi.org/10.1016/j.jcat.2021.06.016
- Chen X. ; Cheng Y. ; Seo C.Y. ; et al . Aging, re-dispersion, and catalytic oxidation characteristics of model Pd/Al 2O3 automotive three-way catalysts . Appl. Catal. B 2015, 163, 499‒ 509. DOI: https://doi.org/10.1016/j.apcatb.2014.08.018
- Heck R.M. ; Farrauto R . J. Automobile exhaust catalysts. Appl. Catal., A 2001, 221, 443‒ 457. DOI: https://doi.org/10.1016/S0926-860X(01)00818-3
- Beale A.M. ; Gao F. ; Lezcano-Gonzalez I. ; et al . Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44( 20), 7371‒ 405. DOI: https://doi.org/10.1039/C5CS00108K
- Han F. ; Sun H. ; Zhao Z. ; et al . Selective Catalytic Reduction of NOx by Methanol on Metal-Free Zeolite with Brønsted and Lewis Acid Pair. ACS Catal. 2022, 12( 4), 2403‒ 2414. DOI: https://doi.org/10.1021/acscatal.1c05624
- Andana T. ; Rappé K.G. ; Nelson N.C. ; et al . Selective catalytic reduction of NOx with NH3 over Ce-Mn oxide and Cu-SSZ-13 composite catalysts – Low temperature enhancement. Appl. Catal. B 2022, 316, 121522. DOI: https://doi.org/10.1016/j.apcatb.2022.121522
- Shan W. ; Yu Y. ; Zhang Y. ; et al . Theory and practice of metal oxide catalyst design for the selective catalytic reduction of NO with NH3. Catal. Today 2021, 376, 292‒ 301. DOI: https://doi.org/10.1016/j.cattod.2020.05.015
- Russell A. ; Epling, Diesel Oxidation Catalysts W.S. . Catal. Rev. 2011, 53( 4), 337‒ 423. DOI: https://doi.org/10.1080/01614940.2011.596429
- Han F. ; Yuan M. ; Chen H . Selective catalytic reduction of NOx with methanol on H-ZSM-5: The effect of extra-framework aluminum. Catal. Today 2020, 355, 443‒ 449. DOI: https://doi.org/10.1016/j.cattod.2019.07.007
- Johnson, T.V.; Joshi, A. Chapter 1 : Review of deNOx Technology for Mobile Applications . In NOx Trap Catalysts and Technologies: Fundamentals and Industrial Applications. Royal Society of Chemistry: Washington DC, USA, 2018; pp. 1- 35. DOI: 10.1039/9781788013239-00001 DOI: https://doi.org/10.1039/9781788013239-00001
- Wu G. ; Liu S. ; Chen Z. ; et al . Promotion effect of alkaline leaching on the catalytic performance over Cu/Fe-SSZ-13 catalyst for selective catalytic reduction of NOx with NH3. J. Taiwan Inst. Chem. Eng. 2022, 134, 104355. DOI: https://doi.org/10.1016/j.jtice.2022.104355
- Wang X. ; Xu Y. ; Qin M. ; et al . Insight into the effects of Cu2+ ions and CuO species in Cu-SSZ-13 catalysts for selective catalytic reduction of NO by NH3. J. Colloid Interface Sci. 2022, 622, 1‒ 10. DOI: https://doi.org/10.1016/j.jcis.2022.04.110
- Liu Q. ; Bian C. ; Ming S. ; et al . The opportunities and challenges of iron-zeolite as NH 3-SCR catalyst in purification of vehicle exhaust . Appl. Catal. A 2020, 607, 117865. DOI: https://doi.org/10.1016/j.apcata.2020.117865
- Liu Z.G. ; Ottinger N.A. ; Cremeens C . M. Vanadium and tungsten release from V-based selective catalytic reduction diesel aftertreatment. Atmos. Environ. 2015, 104, 154‒ 161. DOI: https://doi.org/10.1016/j.atmosenv.2014.12.063
- Wang, D.-y.; Cao, J.-h.; Tan, P.-q.; et al . Full course evolution characteristics of DPF active regeneration under different inlet HC concentrations. Fuel 2022, 310, 122452. DOI: https://doi.org/10.1016/j.fuel.2021.122452
- Bagi S. ; Kamp C.J. ; Sharma V. ; et al . Multiscale characterization of exhaust and crankcase soot extracted from heavy-duty diesel engine and implications for DPF ash. Fuel 2020, 282, 118878. DOI: https://doi.org/10.1016/j.fuel.2020.118878
- Birkhold F. ; Meingast U. ; Wassermann P. ; et al . Modeling and simulation of the injection of urea-water-solution for automotive SCR DeNOx-systems. Appl. Catal. B 2007, 70( 1‒ 4), 119‒ 127. DOI: https://doi.org/10.1016/j.apcatb.2005.12.035
- Dhillon P.S. ; Harold M.P. ; Wang D. ; et al . Enhanced transport in washcoated monoliths: Application to selective lean NOx reduction and ammonia oxidation. Chem. Eng. J. 2019, 377, 119734. DOI: https://doi.org/10.1016/j.cej.2018.08.120
- Dhillon P.S. ; Harold M.P. ; Wang D. ; et al . Modeling and analysis of transport and reaction in washcoated monoliths: Cu-SSZ-13 SCR and dual-layer Cu-SSZ-13 + Pt/Al2O3 ASC. React. Chem. Eng. 2019, 4( 6), 1103‒ 1115. DOI: https://doi.org/10.1039/C8RE00325D
- Gao F. ; Szanyi J . On the hydrothermal stability of Cu/SSZ-13 SCR catalysts. Appl. Catal. A 2018, 560, 185‒ 194. DOI: https://doi.org/10.1016/j.apcata.2018.04.040
- Azzoni M.E. ; Franchi F.S. ; Usberti N. ; et al . Dual-layer AdSCR monolith catalysts: A new solution for NOx emissions control in cold start applications. Appl. Catal. B 2022, 315, 121544. DOI: https://doi.org/10.1016/j.apcatb.2022.121544
- Gao F. ; Peden C . Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8( 4), 140. DOI: https://doi.org/10.3390/catal8040140
- Fu D. ; Park Y. ; Davis M . E. Confinement effects facilitate low-concentration carbon dioxide capture with zeolites. Proc. Natl. Acad. Sci. U. S. A. 2022, 119( 39), e2211544119. DOI: https://doi.org/10.1073/pnas.2211544119






