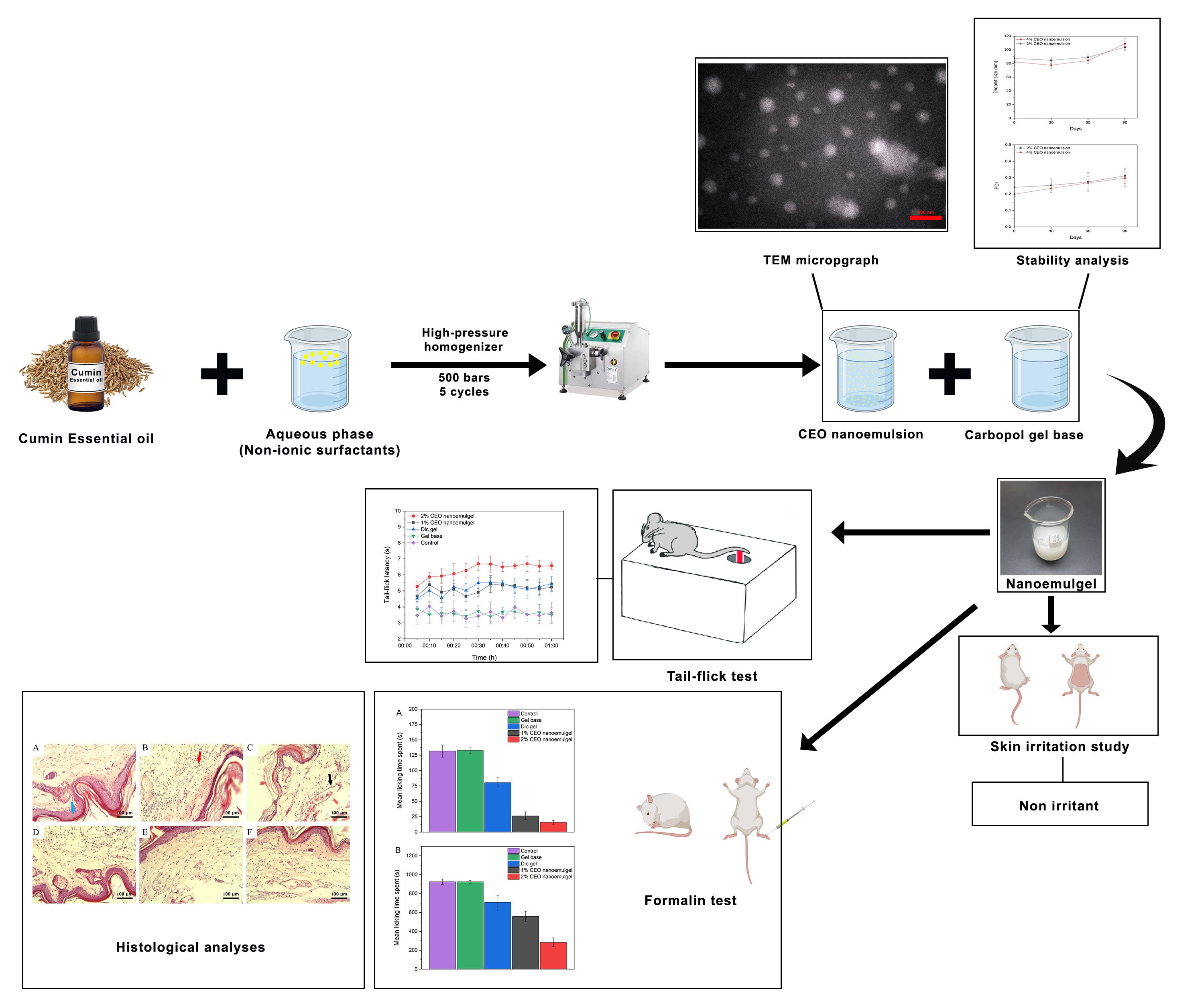
Cuminum cyminum L. (cumin) essential oil (CEO) possesses documented analgesic and anti-inflammatory properties, but its topical application is limited by volatility, instability and low aqueous solubility. This study aimed to develop a CEO nanoemulgel and evaluate its physicochemical characteristics, dermal safety and antinociceptive/anti-inflammatory effects in rodent models. The optimized formulation exhibited nanometric droplet size, uniform distribution and acceptable physical stability. In a rat skin irritation test, repeated application of the CEO nanoemulgel did not produce visible erythema or edema compared with the gel base. In mice, the formulation increased tail-flick latency and reduced nociceptive behaviors in both phases of the formalin test, and histological analysis revealed attenuated inflammatory cell infiltration. These findings suggest that the CEO nanoemulgel has promising antinociceptive and anti-inflammatory activity and appears to be well tolerated in short-term preclinical models. Further studies, including long-term safety assessment, mechanistic analysis and clinical trials, are required before CEO nanoemulgel can be considered for use in humans.
- Open Access
- Article
Cumin Essential Oil Nanoemulgel as a Topical Analgesic and Anti-Inflammatory Alternative to NSAIDs
- Mohammad Eghbali 1,2,
- Majid Saeedi 1,*,
- Katayoun Morteza-Semnani 3,
- Fereshteh Talebpour Amiri 4,
- Jafar Akbari 1,
- Amin Goodarzi 2,3,
- Seyyed Mohammad Hassan Hashemi 5,
- Amirhossein Babaei 2,*
Author Information
Received: 26 Oct 2025 | Revised: 08 Dec 2025 | Accepted: 12 Dec 2025 | Published: 15 Dec 2025
Abstract
Graphical Abstract
Keywords
Cuminum cyminum | antinociceptive | anti-inflammatory | nanoemulgel | essential oils | nanoemulsion
References
- 1.
Sharma, S.; Chala, M.B.; de Jesus-Moraleida, F.R.; et al. Tackling the growing burden of pain in low/middle-income countries. Pain 2025, 166, S140–S145. https://doi.org/10.1097/j.pain.0000000000003672.
- 2.
Stubhaug, A.; Hansen, J.L.; Hallberg, S.; et al. The costs of chronic pain—Long‐term estimates. Eur. J. Pain 2024, 28, 960–977. https://doi.org/10.1002/ejp.2234.
- 3.
Pérez-Martín, Y.; Pérez-Muñoz, M.; Martín-Castro, B.; et al. Exploring Emotional Conflicts and Pain Experience in Patients with Non-Specific Chronic Neck Pain: A Qualitative Study. J. Clin. Med. 2025, 14, 4748. https://doi.org/10.3390/jcm14134748.
- 4.
Fulkerson, M. Pain and psychological integration. Philos. Psychol. 2024, 1–19. https://doi.org/10.1080/09515089.2024.2425338.
- 5.
Quintans-Júnior, L.J.; Brito, R.G.; Quintans, J.S.; et al. Nanoemulsion thermoreversible pluronic F127-based hydrogel containing Hyptis pectinata (Lamiaceae) leaf essential oil produced a lasting anti-hyperalgesic effect in chronic noninflammatory widespread pain in mice. Mol. Neurobiol. 2018, 55, 1665–1675. https://doi.org/10.1007/s12035-017-0438-1.
- 6.
Al Sharie, S.; Varga, S.J.; Al-Husinat, L.i.; et al. Unraveling the complex web of fibromyalgia: A narrative review. Medicina 2024, 60, 272. https://doi.org/10.3390/medicina60020272.
- 7.
Cline, A.E.; Turrentine, J.E. Compounded topical analgesics for chronic pain. Dermatitis 2016, 27, 263–271. https://doi.org/10.1097/DER.0000000000000216.
- 8.
Smith, A.C.; Smith, M.S.; Roach, R.P.; et al. Making Sense of Topical Pain Relief Options: Comparing Topical Analgesics in Efficacy and Safety. Sports Health 2025, 17, 843–852. https://doi.org/10.1177/19417381241280593.
- 9.
Veloso, C.; Cardoso, C.; Vitorino, C. Topical Fixed-Dose Combinations: A Way of Progress for Pain Management? J. Pharm. Sci. 2021, 110, 3345–3361. https://doi.org/10.1016/j.xphs.2021.06.009.
- 10.
Drosopoulou, K.; Kosheleva, R.I.; Ofrydopoulou, A.; et al. Topical and transdermal delivery of nonsteroidal anti-inflammatory drugs (NSAIDs) for inflammation and pain: Current trends and future directions in delivery systems. Processes 2025, 13, 907. https://doi.org/10.3390/pr13030907.
- 11.
Pulskamp, T.G.; Johnson, L.M.; Berlau, D.J. Novel non-opioid analgesics in pain management. Pain Manag. 2024, 14, 641–651. https://doi.org/10.1080/17581869.2024.2442292.
- 12.
Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. https://doi.org/10.1016/j.jconrel.2009.10.016.
- 13.
Ribeiro, H.; Rodrigues, I.; Napoleão, L.; et al. Non-steroidal anti-inflammatory drugs (NSAIDs), pain and aging: Adjusting prescription to patient features. Biomed. Pharmacother. 2022, 150, 112958. https://doi.org/10.1016/j.biopha.2022.112958.
- 14.
Hopkins, S.; Yang, V.; Liew, D.F. Choosing a nonsteroidal anti-inflammatory drug for pain. Aust. Prescr. 2025, 48, 139. https://doi.org/10.18773/austprescr.2025.032.
- 15.
Kopustinskiene, D.M.; Bernatonyte, U.; Maslii, Y.; et al. Natural herbal non-opioid topical pain relievers—Comparison with traditional therapy. Pharmaceutics 2022, 14, 2648. https://doi.org/10.3390/pharmaceutics14122648.
- 16.
Mobasheri, A.; Spring-Charles, A.; Gamaleri, F.C.; et al. Evidence-based opinions from multidisciplinary experts on use of naturopathic herbal remedies in pain management. J. Pain Res. 2024, 17, 599–608. https://doi.org/10.2147/JPR.S432090.
- 17.
Shah, N.; Sapra, R.; Kumari, M.; et al. Herbal Remedies for Pain: A Focus on Plant-Based Solutions. Pharmacogn. Res. 2025, 17, 1127–1131. https://doi.org/10.5530/pres.20252325.
- 18.
Toumia, M.; Dhaoui, R.; Sassi, S.; et al. Efficacy of a natural herbal topical analgesic versus oral paracetamol in patients with soft tissue injury: A randomized, double-blind, placebo-controlled study. Pain Med. 2025, 26, 329–336. https://doi.org/10.1093/pm/pnaf006.
- 19.
Antonelli, A.; Bianchi, M.; Fear, E.J.; et al. Management of Fibromyalgia: Novel Nutraceutical Therapies Beyond Traditional Pharmaceuticals. Nutrients 2025, 17, 530. https://doi.org/10.3390/nu17030530.
- 20.
Patel, M.; Wahezi, S.; Mavrocordatos, P.; et al. The Effects and Mechanisms of Phytochemicals on Pain Management and Analgesic. Nutrients 2025, 17, 633. https://doi.org/10.3390/nu17040633.
- 21.
Sic, A.; Manzar, A.; Knezevic, N.N. The role of phytochemicals in managing neuropathic pain: How much progress have we made? Nutrients 2024, 16, 4342. https://doi.org/10.3390/nu16244342.
- 22.
Borges, R.S.; Lima, E.S.; Keita, H.; et al. Anti-inflammatory and antialgic actions of a nanoemulsion of Rosmarinus officinalis L. essential oil and a molecular docking study of its major chemical constituents. Inflammopharmacology 2018, 26, 183–195. https://doi.org/10.1007/s10787-017-0374-8.
- 23.
Mohammed, H.A.; Sulaiman, G.M.; Al‐Saffar, A.Z.; et al. Aromatic Volatile Compounds of Essential Oils: Distribution, Chemical Perspective, Biological Activity, and Clinical Applications. Food Sci. Nutr. 2025, 13, e70825. https://doi.org/10.1002/fsn3.70825.
- 24.
Johri, R. Cuminum cyminum and Carum carvi: An update. Pharmacogn. Rev. 2011, 5, 63. https://doi.org/10.4103/0973-7847.79101.
- 25.
Sayah, M.; Peirouvi, A.; Kamalinezhad, M. Anti-nociceptive effect of the fruit essential oil of Cuminum cyminum L. in rat. Iran. Biomed. J. 2002, 6, 141–145.
- 26.
Allaq, A.A.; Sidik, N.J.; Abdul-Aziz, A.; et al. Cumin (Cuminum cyminum L.): A review of its ethnopharmacology, phytochemistry. Biomed. Res. Ther. 2020, 7, 4016–4021. https://doi.org/10.15419/bmrat.v7i9.634.
- 27.
Khorasani, S.; Varzi, H.; Ataee, R.; et al. The Effect of Cumin Essential Oil on the Changes in Total Oxidant Status and Interleukin-6 in the Model of Gastric Ulcer in Rats. Glob. J. Medical. Clin. Case Rep. 2024, 12, 117–120. https://doi.org/10.17352/2455-5282.000211.
- 28.
Shaheen, H.M.; Smlkb, B.; Nyemb, J.N.; et al. Ethnomedicinal uses, phytochemical, pharmacological and pharmacokinetics properties of Cumin (Cuminum cyminum). J. Phytopharm. 2023, 12, 315–325. https://doi.org/10.31254/phyto.2023.12507.
- 29.
Paul, S.; El Bethel Lalthavel Hmar, J.H.; Zothantluanga, H.K.S. Essential oils: A review on their salient biological activities and major delivery strategies. Sci. Vis. 2020, 20, 54–71. https://doi.org/10.33493/scivis.20.02.01.
- 30.
Chen, J.; Jiang, Q.-D.; Wu, Y.-M.; et al. Potential of essential oils as penetration enhancers for transdermal administration of ibuprofen to treat dysmenorrhoea. Molecules 2015, 20, 18219–18236. https://doi.org/10.3390/molecules201018219.
- 31.
Kaspute, G.; Ivaskiene, T.; Ramanavicius, A.; et al. Terpenes and Essential Oils in Pharmaceutics: Applications as Therapeutic Agents and Penetration Enhancers with Advanced Delivery Systems for Improved Stability and Bioavailability. Pharmaceutics 2025, 17, 793. https://doi.org/10.3390/pharmaceutics17060793.
- 32.
Yihan, W.; Jinjin, D.; Yingqi, W.; et al. Advances in plant essential oils and drug delivery systems for skincare. Front. Pharmacol. 2025, 16, 1578280. https://doi.org/10.3389/fphar.2025.1578280.
- 33.
de Matos, S.P.; Lucca, L.G.; Koester, L.S. Essential oils in nanostructured systems: Challenges in preparation and analytical methods. Talanta 2019, 195, 204–214. https://doi.org/10.1016/j.talanta.2018.11.029.
- 34.
Kshyap, N.; Kumari, A.; Raina, N.; et al. Prospects of essential oil loaded nanosystems for skincare. Phytomed. Plus 2021, 2, 100198. https://doi.org/10.1016/j.phyplu.2021.100198.
- 35.
Yap, X.F.; Saw, S.H.; Lim, V.; et al. Plant essential oil nanoemulgel as a cosmeceutical ingredient: A review. Cosmetics 2024, 11, 116. https://doi.org/10.3390/cosmetics11040116.
- 36.
Mohammadifar, M.; Talaei, S.A.; Vakili, Z.; et al. Eavaluation of antinociceptic effect of nano-emulsion gel conataining rosemary and peppermint essential oils in a rat model of osteoarthritis. Sci. J. Kurd. Univ. Med. Sci. 2018, 23, 100–109.
- 37.
Lal, D.K.; Kumar, B.; Saeedan, A.S.; et al. An Overview of Nanoemulgels for Bioavailability Enhancement in Inflammatory Conditions via Topical Delivery. Pharmaceutics 2023, 15, 1187. https://doi.org/10.3390/pharmaceutics15041187.
- 38.
Omidian, H.; Cubeddu, L.X.; Gill, E.J. Harnessing nanotechnology to enhance essential oil applications. Molecules 2025, 30, 520. https://doi.org/10.3390/molecules30030520.
- 39.
Chatzidaki, M.D.; Mitsou, E. Advancements in nanoemulsion-based drug delivery across different administration routes. Pharmaceutics 2025, 17, 337. https://doi.org/10.3390/pharmaceutics17030337.
- 40.
Ansari, A.; Verma, M.; Majhi, S. Nanoemulgel: A Comprehensive Review of Formulation Strategies, Characterization, Patents and Applications. Micro Nanosyst. 2025, 17, 12–26. https://doi.org/10.2174/0118764029316500240904074317.
- 41.
Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. https://doi.org/10.1111/jphp.12334.
- 42.
Lohani, A.; Verma, A.; Joshi, H.; et al. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687.
- 43.
Bahloul, B.; Ben Bnina, E.; Hamdi, A.; et al. Investigating the wound-healing potential of a nanoemulsion–gel formulation of pituranthos tortuosus essential oil. Gels 2024, 10, 155. https://doi.org/10.3390/gels10030155.
- 44.
Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives-Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. https://doi.org/10.3390/ijms21197078.
- 45.
Fouda, K.; Mohamed, R.S. In Silico and In Vivo Studies Reveal the Potential Preventive Impact of Cuminum cyminum and Foeniculum vulgare Essential Oil Nanocapsules Against Depression-like States in Mice Fed a High-Fat Diet and Exposed to Chronic Unpredictable Mild Stress. Sci. Pharm. 2025, 93, 37. https://doi.org/10.3390/scipharm93030037.
- 46.
de Matos, S.P.; Teixeira, H.F.; De Lima, Á.A.; et al. Essential oils and isolated terpenes in nanosystems designed for topical administration: A review. Biomolecules 2019, 9, 138. https://doi.org/10.3390/biom9040138.
- 47.
Morteza-Semnani, K.; Saeedi, M.; Akbari, J.; et al. Development of a novel nanoemulgel formulation containing cumin essential oil as skin permeation enhancer. Drug Deliv. Transl. Res. 2022, 12, 1455–1465. https://doi.org/10.1007/s13346-021-01025-1.
- 48.
Rodrigues, F.V.; Diniz, L.S.; Sousa, R.M.; et al. Preparation and characterization of nanoemulsion containing a natural naphthoquinone. Quim. Nova 2018, 41, 756–761. https://doi.org/10.21577/0100-4042.20170247.
- 49.
Ullah, N.; Amin, A.; Farid, A.; et al. Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections. Gels 2023, 9, 252. https://doi.org/10.3390/gels9030252.
- 50.
Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; et al. An Eco-Friendly and Hopeful Promise Platform for Delivering Hydrophilic Wound Healing Agents in Topical Administration for Wound Disorder: Diltiazem-Loaded Niosomes. J. Pharm. Innov. 2023, 18, 1111–1127. https://doi.org/10.1007/s12247-023-09710-z.
- 51.
Akbari, J.; Saeedi, M.; Farzin, D.; et al. Transdermal absorption enhancing effect of the essential oil of Rosmarinus officinalis on percutaneous absorption of Na diclofenac from topical gel. Pharm. Biol. 2015, 53, 1442–1447. https://doi.org/10.3109/13880209.2014.984855.
- 52.
Çağlar, E.Ş.; Okur, M.E.; Aksu, B.; et al. Transdermal delivery of acemetacin loaded microemulsions: Preparation, characterization, in vitro–ex vivo evaluation and in vivo analgesic and anti-inflammatory efficacy. J. Dispers. Sci. Technol. 2023, 45, 662–672. https://doi.org/10.1080/01932691.2023.2175691.
- 53.
Hacısüleyman, L.; Saraç, B.; Joha, Z. Analgesic Effects of Vilazodone, Indatraline, and Talsupram in a Rat Model of Neuropathic Pain. Turk J. Pharm. Sci. 2022, 19, 336–342. https://doi.org/10.4274/tjps.galenos.2021.41514.
- 54.
Afridi, H.H.; Shoaib, M.; Al-Joufi, F.A.; et al. Synthesis and Investigation of the Analgesic Potential of Enantiomerically Pure Schiff Bases: A Mechanistic Approach. Molecules 2022, 27, 5206. https://doi.org/10.3390/molecules27165206.
- 55.
Khalouzadeh, F.; Azizi, H.; Semnanian, S. Adolescent nicotine exposure increases nociceptive behaviors in rat model of formalin test: Involvement of ventrolateral periaqueductal gray neurons. Life Sci. 2022, 299, 120551. https://doi.org/10.1016/j.lfs.2022.120551.
- 56.
Sadeghi-Ghadi, Z.; Ebrahimnejad, P.; Talebpour Amiri, F.; et al. Improved oral delivery of quercetin with hyaluronic acid containing niosomes as a promising formulation. J. Drug Target. 2021, 29, 225–234. https://doi.org/10.1080/1061186X.2020.1830408.
- 57.
Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. https://doi.org/10.1038/srep42717.
- 58.
Banerjee, P.; Kemmler, E.; Dunkel, M.; et al. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. https://doi.org/10.1093/nar/gkae303.
- 59.
Fu, L.; Shi, S.; Yi, J.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, W422–W431. https://doi.org/10.1093/nar/gkae236.
- 60.
Romes, N.B.; Abdul Wahab, R.; Abdul Hamid, M.; et al. Thermodynamic stability, in-vitro permeability, and in-silico molecular modeling of the optimal Elaeis guineensis leaves extract water-in-oil nanoemulsion. Sci. Rep. 2021, 11, 20851. https://doi.org/10.1038/s41598-021-00409-0.
- 61.
Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. https://doi.org/10.1023/a:1015810312465.
- 62.
Morteza-Semnani, K.; Ahadi, H.; Hashemi, Z. The genus Hymenocrater: A comprehensive review. Pharm. Biol. 2016, 54, 3156–3163. https://doi.org/10.1080/13880209.2016.1197285.
- 63.
Passos, F.; Lopes, E.; de Araújo, J.; et al. Involvement of Cholinergic and Opioid System in γ-Terpinene-Mediated Antinociception. Evid. Based Complement. Alternat. Med. 2015, 2015, 829414. https://doi.org/10.1155/2015/829414.
- 64.
Koohsari, S.; Sheikholeslami, M.A.; Parvardeh, S.; et al. Antinociceptive and antineuropathic effects of cuminaldehyde, the major constituent of Cuminum cyminum seeds: Possible mechanisms of action. J. Ethnopharmacol. 2020, 255, 112786. https://doi.org/10.1016/j.jep.2020.112786.
- 65.
Ramalho, T.R.; Oliveira, M.T.; Lima, A.L.; et al. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015, 81, 1248–1254. https://doi.org/10.1055/s-0035-1546169.
- 66.
Tomy, M.; Dileep, K.; Prasanth, S.; et al. Cuminaldehyde as a Lipoxygenase Inhibitor: In Vitro and in Silico Validation. Appl. Biochem. Biotechnol. 2014, 174, 388–397. https://doi.org/10.1007/s12010-014-1066-0.
- 67.
de Santana, M.F.; Guimarães, A.G.; Chaves, D.O.; et al. The anti-hyperalgesic and anti-inflammatory profiles of p-cymene: Evidence for the involvement of opioid system and cytokines. Pharm. Biol. 2015, 53, 1583–1590. https://doi.org/10.3109/13880209.2014.993040.
- 68.
Bonjardim, L.R.; Cunha, E.S.; Guimarães, A.G.; et al. Evaluation of the anti-inflammatory and antinociceptive properties of p-cymene in mice. Z. Für Naturforschung C J. Biosci. 2012, 67, 15–21. https://doi.org/10.1515/znc-2012-1-203.
- 69.
Santana, M.F.; Quintans-Júnior, L.J.; Cavalcanti, S.C.; et al. p-Cymene reduces orofacial nociceptive response in mice. Rev. Bras. Farmacogn. 2011, 21, 1138–1143. https://doi.org/10.1590/S0102-695X2011005000156.
- 70.
Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models. ISRN Toxicol. 2013, 2013, 459530. https://doi.org/10.1155/2013/459530.
- 71.
Paul, A.; Mishra, S.S.; Maji, A.; et al. Exploring the therapeutic potentials of cuminaldehyde: A comprehensive review of biological activities, mechanisms, and novel delivery systems. Phytochem. Rev. 2025, 24, 5207–5238. https://doi.org/10.1007/s11101-025-10069-x.
- 72.
Azubugwu, C.M.; Feridious, N.; Mostafa, F.; et al. Evaluation of phytochemical, analgesic, acute toxicity and moisture content of Cumin cyminum. World News Nat. Sci. 2024, 52, 82–97.
- 73.
Gheisary, B.; Ashrafi-Saeidlou, S.; Hassani, A.; et al. Enhancing antioxidant and antibacterial activities of Cuminum cyminum, Origanum vulgare, and Salvia officinalis essential oils through a synergistic perspective. Sci. Rep. 2025, 15, 26728. https://doi.org/10.1038/s41598-025-10814-4.
- 74.
Ladan Moghadam, A.R. Chemical composition and antioxidant activity Cuminum cyminum L. essential oils. Int. J. Food Prop. 2016, 19, 438–442. https://doi.org/10.1080/10942912.2015.1038355.
- 75.
Moghaddam, M.; Miran, S.N.K.; Pirbalouti, A.G.; et al. Variation in essential oil composition and antioxidant activity of cumin (Cuminum cyminum L.) fruits during stages of maturity. Ind. Crops Prod. 2015, 70, 163–169. https://doi.org/10.1016/j.indcrop.2015.03.031.
- 76.
Gambini, J.; Stromsnes, K. Oxidative Stress and Inflammation: From Mechanisms to Therapeutic Approaches. Biomedicines 2022, 10, 753. https://doi.org/10.3390/biomedicines10040753.
- 77.
Liu, F.-L.; Fang, S.-Y.; Chan, H.-W.; et al. Formulation and evaluation of ulvan-functionalized cumin oil nanoemulsions for antioxidant, anti-photoaging, and anti-melanogenesis activities in skin cells. Bioresour. Bioprocess. 2025, 12, 137. https://doi.org/10.1186/s40643-025-00980-8.
- 78.
Nouri, A.; Mofasseri, M.; Jahani, R.; et al. Phytochemical composition, hypnotic activity, and antinociceptive properties of cumin essential oil collected from various geographical regions. Food Sci. Nutr. 2024, 12, 9025–9034. https://doi.org/10.1002/fsn3.4432.
- 79.
Ranjbar, R.; Zarenezhad, E.; Abdollahi, A.; et al. Nanoemulsion and nanogel containing Cuminum cyminum L. essential oil: Antioxidant, anticancer, antibacterial, and antilarval properties. J. Trop. Med. 2023, 2023, 5075581. https://doi.org/10.1155/2023/5075581.
- 80.
El Asbahani, A.; Miladi, K.; Badri, W.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. https://doi.org/10.1016/j.ijpharm.2014.12.069.
- 81.
Eid, A.M.; El-Enshasy, H.A.; Aziz, R.; et al. Preparation, characterization and anti-inflammatory activity of Swietenia macrophylla nanoemulgel. J. Nanomed. Nanotechnol. 2014, 5, 1–10. https://doi.org/10.4172/2157-7439.1000190.
- 82.
Jeengar, M.K.; Rompicharla, S.V.K.; Shrivastava, S.; et al. Emu oil based nano-emulgel for topical delivery of curcumin. Int. J. Pharm. 2016, 506, 222–236. https://doi.org/10.1016/j.ijpharm.2016.04.052.
- 83.
Esmaeili, F.; Zahmatkeshan, M.; Yousefpoor, Y.; et al. Anti-inflammatory and anti-nociceptive effects of Cinnamon and Clove essential oils nanogels: An in vivo study. BMC Complement. Med. Ther. 2022, 22, 143. https://doi.org/10.1186/s12906-022-03619-9.
- 84.
Moawad, S.; Badr, A.N.; Farouk, A.; et al. Bioactivity and Nanoformulation of Eucalyptus camaldulensis Essential Oils: Implications for Antioxidant and Anti-inflammatory Applications. ACS Omega 2025, 10, 26729–26742. https://doi.org/10.1021/acsomega.5c01246.
- 85.
Shehata, T.M.; Elnahas, H.M.; Elsewedy, H.S. Development, Characterization and Optimization of the Anti-Inflammatory Influence of Meloxicam Loaded into a Eucalyptus Oil-Based Nanoemulgel. Gels 2022, 8, 262. https://doi.org/10.3390/gels8050262.
- 86.
Alhakamy, N.A.; Kotta, S.; Ali, J.; et al. Formulation Development, Statistical Optimization, In Vitro and In Vivo Evaluation of Etoricoxib-Loaded Eucalyptus Oil-Based Nanoemulgel for Topical Delivery. Appl. Sci. 2021, 11, 7294. https://doi.org/10.3390/app11167294.
- 87.
Ali, B.; Al-Wabel, N.A.; Shams, S.; et al. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. https://doi.org/10.1016/j.apjtb.2015.05.007.
- 88.
Oliveira, R.; Almeida, I.F. Patient-Centric Design of Topical Dermatological Medicines. Pharmaceuticals 2023, 16, 617. https://doi.org/10.3390/ph16040617.
- 89.
Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; et al. Application of essential oils as preservatives in food systems: Challenges and future prospectives—A review. Phytochem. Rev. 2022, 21, 1209–1246. https://doi.org/10.1007/s11101-021-09776-y.
- 90.
Organization, W.H. WHO Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems; World Health Organization: Geneva, Switzerland, 2004.

This work is licensed under a Creative Commons Attribution 4.0 International License.


