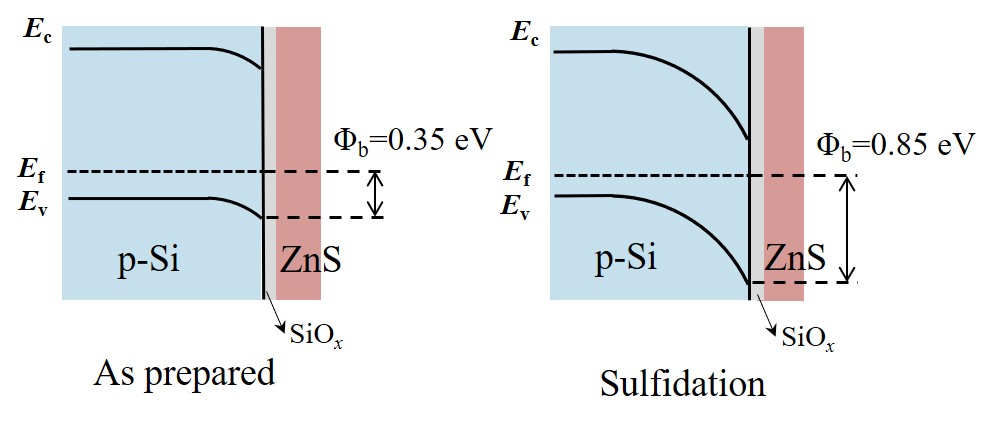
Silicon (Si) is considered one of the most promising semiconductor materials for photocathodes in photoelectrochemical (PEC) hydrogen production, owing to its narrow bandgap, excellent optoelectronic properties, and earth abundance. To achieve efficient charge separation and transport during PEC operation, selecting an appropriate electron transport layer (ETL) on p-Si is essential for constructing a buried junction. However, the development of p-Si heterojunction photocathodes with high photovoltage and efficient interfacial carrier transfer is often hindered by unfavorable band alignment between the absorber and ETL. In this study, an ultrathin ZnS layer was employed as the ETL for p-Si due to its low electron affinity, which enables a high theoretical photovoltage for the heterojunction photocathode. Furthermore, a sulfidation process was applied to passivate the ZnS layer. After sulfidation at 400 °C, the p-Si/ZnS/Pt photocathode exhibited a positive onset potential of 0.5 VRHE and an ABPE of 2.3% at 0.2 VRHE. EDS and XPS analyses confirmed the removal of oxidized species and the formation of sulfur vacancies in suitable amounts, which led to optimized carrier concentration and barrier height, thereby enhancing charge transfer kinetics and suppressing carrier recombination.
- Open Access
- Article
Ultrathin ZnS Electron Transport Layer Enables Efficient p-Si Photocathode for Photoelectrochemical Hydrogen Production
- Yanming Li 1,†,
- Jianjun Jiang 1,†,
- Chenglong Ding 1,
- Hao Wang 1,
- Yequan Xiao 2,*,
- Jingfu He 1,*,
- Changli Li 1,*
Author Information
Received: 28 Sep 2025 | Revised: 04 Nov 2025 | Accepted: 10 Nov 2025 | Published: 17 Nov 2025
Abstract
Graphical Abstract
Keywords
p-Si photocathode | ultrathin ZnS layer | sulfidation | sulfur vacancies | PEC hydrogen production
References
- 1.Li, C.; He, J.; Xiao, Y.; et al. Earth-Abundant Cu-Based Metal Oxide Photocathodes for Photoelectrochemical Water Splitting. Energy Environ. Sci. 2020, 13, 3269–3306.
- 2.Feng, C.; Faheem, M.B.; Fu, J.; et al. Fe-Based Electrocatalysts for Oxygen Evolution Reaction: Progress and Perspectives. ACS Catal. 2020, 10, 4019–4047.
- 3.Huang, Q.; Ye, Z.; Xiao, X. Recent Progress in Photocathodes for Hydrogen Evolution. J. Mater. Chem. A 2015, 3, 15824–15837.
- 4.
Xiao, W.; Lin, H.; Lu, L.; et al. Elucidating the Origin of Positive Onset Potential and Low Photovoltage of CuBi2O4 Photocathode. J. Catal. 2025, 450, 116325.
- 5.Cheng, C.; Zhang, W.; Chen, X.; et al. Strategies for improving photoelectrochemical water splitting performance of Si—based electrodes. Energy Sci. Eng. 2022, 10, 1526–1543.
- 6.Li, Y.; Xiao, Y.; Wu, C.; et al. Strategies To Construct N-Type Si-Based Heterojunctions for Photoelectrochemical Water Oxidation. ACS Mater. Lett. 2022, 4, 779–804.
- 7.Wang, H.-P.; Sun, K.; Noh, S.Y.; et al. High-Performance a-Si/c-Si Heterojunction Photoelectrodes for Photoelectrochemical Oxygen and Hydrogen Evolution. Nano Lett. 2015, 15, 2817–2824.
- 8.
Ji, L.; McDaniel, M.D.; Wang, S.; et al. A silicon-based photocathode for water reduction with an epitaxial SrTiO3 protection layer and a nanostructured catalyst. Nat. Nanotechnol. 2014, 10, 84–90.
- 9.
Li, Y.; Ding, C.; Li, Y.; et al. Engineering the SiOX Interfacial Layer of Si-Based Metal-Insulator-Semiconductor Junction for Photoelectrochemical Hydrogen Production. J. Catal. 2024, 434, 115533.
- 10.Li, Y.; Ding, C.; Li, Y.; et al. Engineering the Inhomogeneity of Metal–Insulator–Semiconductor Junctions for Photoelectrochemical Methanol Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 59403–59412.
- 11.
Zhang, D.; Liang, W.; Sharma, A.; et al. Ultrathin HfO2 Passivated Silicon Photocathodes for Efficient Alkaline Water Splitting. Appl. Phys. Lett. 2021, 119, 193901.
- 12.Sharma, A.; Duong, T.; Liu, P.; et al. Direct Solar to Hydrogen Conversion Enabled by Silicon Photocathodes with Carrier Selective Passivated Contacts. Sustain. Energy Fuels 2022, 6, 349–360.
- 13.Pérez-del-Rey, D.; Boix, P.P.; Sessolo, M.; et al. Interfacial Modification for High-Efficiency Vapor-Phase-Deposited Perovskite Solar Cells Based on a Metal Oxide Buffer Layer. J. Phys. Chem. Lett. 2018, 9, 1041–1046.
- 14.Moon, D.G.; Rehan, S.; Yeon, D.H.; et al. A Review on Binary Metal Sulfide Heterojunction Solar Cells. Sol. Energy Mater. Sol. Cells 2019, 200, 109963.
- 15.Yi, G.; Wang, Q.; Arbiol, J.; et al. Emerging Metal Oxide/Nitride Protection Layers for Enhanced Stability of Silicon Photoelectrodes in Photoelectrochemical Catalysis: Recent Advancements and Challenges. Mater. Today Chem. 2023, 34, 101795.
- 16.
Wang, J.; Lin, S.; Tian, N.; et al. Nanostructured Metal Sulfides: Classification, Modification Strategy, and Solar—Driven CO2 Reduction Application. Adv. Funct. Mater. 2020, 31, 2008008.
- 17.
Chae, S.Y.; Park, S.J.; Han, S.G.; et al. Enhanced Photocurrents with ZnS Passivated Cu(In,Ga)(Se,S)2 Photocathodes Synthesized Using a Nonvacuum Process for Solar Water Splitting. J. Am. Chem. Soc. 2016, 138, 15673–15681.
- 18.
Wang, K.; Huang, D.; Yu, L.; et al. Environmentally Friendly Cu2ZnSnS4-Based Photocathode Modified with a ZnS Protection Layer for Efficient Solar Water Splitting. J. Colloid Interface Sci. 2019, 536, 9–16.
- 19.Patel, S.L.; Purohit, A.; Chander, S.; et al. Thermal Annealing Evolution to Physical Properties of ZnS Thin Films as Buffer Layer for Solar Cell Applications. Phys. E Low Dimens. Syst. Nanostructures 2018, 101, 174–177.
- 20.Chen, S.; Song, L.; Zhang, P.; et al. Influence of Low-Temperature Sulfidation on the Structure of ZnS Thin Films. Chin. Phys. B 2019, 28, 024214.
- 21.Sharma, K.; Kumar, A.; Ahamad, T.; et al. Sulphur Vacancy Defects Engineered Metal Sulfides for Amended Photo (Electro) Catalytic Water Splitting: A Review. J. Mater. Sci. Technol. 2023, 152, 50–64.
- 22.Dai, P.; Li, W.; Xie, J.; et al. Forming Buried Junctions to Enhance the Photovoltage Generated by Cuprous Oxide in Aqueous Solutions. Angew. Chem. 2014, 126, 13711–13715.
- 23.Buckley, A.N.; Wouterlood, H.J.; Woods, R. The Surface Composition of Natural Sphalerites under Oxidative Leaching Conditions. Hydrometallurgy 1989, 22, 39–56.
- 24.
Agostinelli, E.; Battistoni, C.; Fiorani, D.; et al. An XPS Study of the Electronic Structure of the ZnXCd1−XCr2(X = S, Se) Spinel System. J. Phys. Chem. Solids 1989, 50, 269–272.
- 25.
Hu, S.; Jin, L.; Si, W.; et al. Sulfur Vacancies Enriched 2D ZnIn2S4 Nanosheets for Improving Photoelectrochemical Performance. Catalysts 2022, 12, 400.
- 26.Loget, G.; Mériadec, C.; Dorcet, V.; et al. Tailoring the photoelectrochemistry of catalytic metal-insulator-semiconductor (MIS) photoanodes by a dissolution method. Nat. Commun. 2019, 10, 3522.
- 27.
Zhang, D.; Li, H.; Riaz, A.; et al. Unconventional Direct Synthesis of Ni3N/Ni with N-Vacancies for Efficient and Stable Hydrogen Evolution. Energy Environ. Sci. 2022, 15, 185–195.
- 28.Zhang, D.; Pan, W.; Sharma, A.; et al. Over 14% Unassisted Water Splitting Driven by Immersed Perovskite/Si Tandem Photoanode with Ni-Based Catalysts. Mater. Today Energy 2025, 48, 101809.
- 29.Streetman, B.G.; Banerjee, S. Solid State Electronic Devices New Jersey; Prentice Hall: Upper Saddle River, NJ, USA, 2000.
- 30.
Wang, L.; Xia, L.; Wu, Y.; et al. Zr-Doped β-In2S3 Ultrathin Nanoflakes as Photoanodes: Enhanced Visible-Light-Driven Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2016, 4, 2606–2614.
- 31.
Cheiwchanchamnangij, T.; Lambrecht, W.R. Quasiparticle band structure calculation of monolayer, bilayer, and bulk MoS2. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85, 205302.
- 32.
Zhao, Z.; Cao, Y.; Yi, J.; et al. Band-Edge Electronic Structure of β-In2S3: The Role of s or p Orbitals of Atoms at Different Lattice Positions. ChemPhysChem 2012,13, 1551–1556.

This work is licensed under a Creative Commons Attribution 4.0 International License.