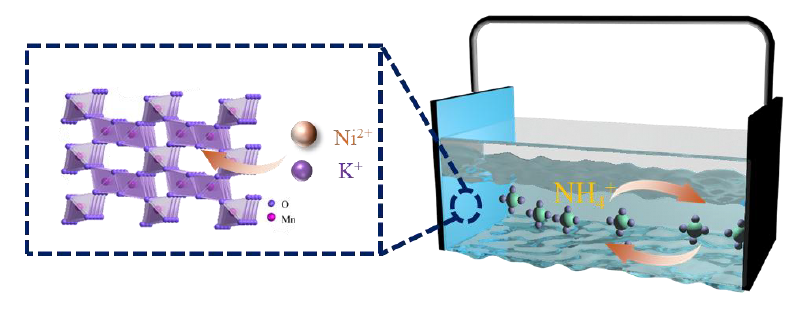
In ammonium-ion batteries, manganese dioxide (MnO2) exhibits promising ammonium storage capabilities but suffers from inherent limitations such as poor electrical conductivity and structural instability. To address these challenges, this study proposes a cation doping strategy involving individual (Ni2+ or K+) and dual doping strategies. The incorporation of these dopants significantly enhances the ammonium storage performance of MnO2. Single doping (Ni2+ or K+) and dual doping strategies significantly improve the ammonium storage performance of MnO2. Ni2+ doping induces oxygen vacancies and modulates the electronic structure, extending the cycling lifespan to 727 cycles (60% capacity retention) and reducing charge transfer resistance to 9.42 Ω. K+ doping forms a KMn8O16 phase with [2 × 2] tunnel structures, elevating the NH4+ diffusion coefficient to 10−10~10−9 cm2 s−1 and improving rate capability (73.75 mAh g−1 at 1 A g−1). The dual-doped system (NiK-MnO2) achieves a specific capacity of 165.94 mAh g−1 at 0.1 A g−1 through synergistic effects. It maintains 60% capacity retention after 917 cycles at 1 A g−1 with an impedance of 9.08 Ω and a resistivity of 3.02 mΩ cm. Dual doping improves the electrical conductivity of the electrode material and enhances the ammonium storage performance of MnO2 through a synergistic mechanism of oxygen vacancies and tunneling structure.
- Open Access
- Article
Engineering Oxygen Vacancies and Ion Diffusion Channels via Ni2+/K+ Co-Doping for Aqueous Ammonium-Ion Batteries
- Xiaoqi Tang 1,†,
- Nannan Zhong 1,†,
- Dan Li 2,
- Jingyi Guan 1,
- Ning Cao 1,
- Xiaobei Zang 1,*,
- Qingguo Shao 3,*
Author Information
Received: 09 Oct 2025 | Revised: 07 Nov 2025 | Accepted: 10 Nov 2025 | Published: 13 Nov 2025
Abstract
Graphical Abstract
References
- 1.
Han, J.; Varzi, A.; Passerini, S. The emergence of aqueous ammonium‐ion batteries. Angew. Chem. 2022, 134, e202115046.
- 2.
Pan, Y.; Yuan, L.; Liu, L.; et al. Critical advances of aqueous rechargeable ammonium ion batteries. Small Struct. 2023, 4, 2300201.
- 3.
Zhang, R.; Wang, S.; Chou, S.; et al. Research development on aqueous ammonium‐ion batteries. Adv. Funct. Mater. 2022, 32, 2112179.
- 4.
Ao, H.; Zhu, W.; Liu, M.; et al. High‐Voltage and Super‐Stable Aqueous Sodium–Zinc Hybrid Ion Batteries Enabled by Double Solvation Structures in Concentrated Electrolyte. Small Methods 2021, 5, 2100418.
- 5.
Zhang, Q.; Luan, J.; Tang, Y.; et al. Interfacial design of dendrite‐free zinc anodes for aqueous zinc‐ion batteries. Angew. Chem. Int. Ed. 2020, 59, 13180–13191.
- 6.
Hu, F.; Xie, D.; Zhao, D.; et al. Na2V6O16·2.14H2O nanobelts as a stable cathode for aqueous zinc-ion batteries with long-term cycling performance. J. Energy Chem. 2019, 38, 185–191.
- 7.
Zeng, Y.; Yang, J.; Yang, H.; et al. Bridging microstructure and sodium-ion storage mechanism in hard carbon for sodium ion batteries. ACS Energy Lett. 2024, 9, 1184–1191.
- 8.
Wang, Q.; Zhou, D.; Zhao, C.; et al. Fast-charge high-voltage layered cathodes for sodium-ion batteries. Nat. Sustain. 2024, 7, 338–347.
- 9.
Sun, T.; Yao, X.; Luo, Y.; et al. Micron-sized Na0.7MnO2.05 as cathode materials for aqueous rechargeable magnesium-ion batteries. Ionics 2019, 25, 4805–4815.
- 10.
You, H.; Yang, C.; Liu, Z.; et al. Heterojunction of Vanadium Oxide Nanobelts for Aqueous Magnesium-Ion Batteries. ACS Sustain. Chem. Eng. 2025, 13, 890–897.
- 11.
Liang, G.; Mo, F.; Ji, X.; et al. Non-metallic charge carriers for aqueous batteries. Nat. Rev. Mater. 2021, 6, 109–123.
- 12.
Yu, D.; Tian, R.; Du, F. Recent advances in aqueous batteries with nonmetal cations as charge carriers. Adv. Energy Sustain. Res. 2022, 3, 2100207.
- 13.
Wang, Y.; Kuchena, S.F. Recent progress in aqueous ammonium-ion batteries. ACS Omega 2022, 7, 33732–33748.
- 14.
Lu, Y.; Wang, T.; Naresh, N.; et al. Pre-doped cations in V2O5 for high-performance Zn-ion batteries. Nano Res. Energy 2024, 3, e9120125.
- 15.
Lu, T.H.; Liu, Q.; He, J.; et al. Ethanediamine Intercalation Induced Hydrogen Bond Network in Vanadium Oxide for Ultralong‐Life Aqueous Ammonium Ion Batteries. Batter. Supercaps 2025, 8, e202400426.
- 16.
Wang, Y. Tailoring Electrodes and Electrolytes in Emerging Aqueous Ammonium-Ion Batteries for Enhanced Performance. In Electrochemical Society Meeting Abstracts 241; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2022.
- 17.
Zheng, R.; Li, Y.; Yu, H.; et al. Ammonium ion batteries: Material, electrochemistry and strategy. Angew. Chem. 2023, 135, e202301629.
- 18.
Uçan, M.; Tekin, B. Toward a Safer and Greener Future: Reliable Aqueous Ammonium-Ion Batteries with LiMnO2 Cathodes. Bitlis Eren Üniversitesi Fen Bilim. Derg. 2025, 14, 1787–1801.
- 19.
Uçan, M.; Topcu, Y.; Tekin, B. First-Ever Use of LiMn2O4 Cathode in State-of-the-Art Ammonium-Ion Batteries: Unlocking a New Ametal Charge Carrier. Erzincan Univ. J. Sci. Technol. 2025, 18, 431–450.
- 20.
Bao, S.-J.; Jia, W.; Lei, C.; et al. Preparation and Electrochemical Capacitor Properties of Novel Birnessite-Type Manganese Dioxide. In ECS Meeting Abstracts; IOP Publishing: Jefferson City, MO, USA, 2012; p. 117.
- 21.
Wang, M.; Yagi, S. Layered birnessite MnO2 with enlarged interlayer spacing for fast Mg-ion storage. J. Alloys Compd. 2020, 820, 153135.
- 22.
Song, X.; Wang, H.; Li, Z.; et al. A review of MnO2 composites incorporated with conductive materials for energy storage. Chem. Rec. 2022, 22, e202200118.
- 23.
Salmani, M.R.M.; Mirkazemi, S.M.; Rezaie, H. The effect of Ni2+ and Co2+ dopants on the structural, electrical and optical properties of Sn2−x−yNixCoyO2−zFz thin films. Appl. Phys. A 2025, 131, 531.
- 24.
Miao, G.; Wang, Z.; Sun, F.; et al. Modulating the interlayer H+ migration in MnO2 via W and K co-doping engineering to high-capacity aqueous zinc-ion batteries. J. Colloid Interface Sci. 2025, 693, 137636.
- 25.
Wu, Y.; Xu, L.; Zhong, J.; et al. K+ doping-induced pillaring effect in Na3V2(PO4)3 for enhanced rate performance in sodium-ion batteries. Chem. Commun. 2025, 61, 13445–13448.
- 26.
Xu, J.-H.; Zhu, Y.-H.; Yang, W.-M.; et al. K doping stabilizes three-dimensional K0.2Na1.3Mn0.5O2−δ as high-performance cathode for sodium-ion batteries. Rare Met. 2024, 43, 5030–5038.
- 27.
Yue, Z. Hydrothermal Synthesis of MgAlZnFeCe Hydrotalcite-like Precursors and Their Complex Oxides for Application in FCC De-SOx. Wuji Cailiao Xuebao J. Inorg.Mater. 2009, 24, 171–174.
- 28.
Gu, H.; Wang, F.; Chen, S.; et al. Suppressing Jahn-Teller distortion of MnO2 via B-Ni dual single-atoms integration for methane catalytic combustion. Nat. Commun. 2025, 16, 1008.
- 29.
Tan, B.; Chen, N.; Huang, L.; et al. Enhanced the electrochemical performance of Ni-doped α-MnO2 prepared with one-pot process for supercapacitors. J. Ind. Eng. Chem. 2025, 141, 319–327.
- 30.
Yang, S.; Li, F.; Fu, P.; et al. Nickel-doped δ-MnO2 abundant in oxygen vacancies as a cathode material for aqueous Zn-ion batteries with superior performance. Nanoscale 2025, 17, 7423–7433.
- 31.
Raskar, N.D.; Dake, D.V.; Mane, V.A.; et al. Designing reduced graphene oxide decorated Ni doped δ-MnO2 nanocomposites for supercapacitor applications. Mater. Sci. Semicond. Process. 2024, 178, 108451.
- 32.
Gou, L.; Li, J.; Liang, K.; et al. Bi‐MOF Modulating MnO2 Deposition Enables Ultra‐Stable Cathode‐Free Aqueous Zinc‐Ion Batteries. Small 2023, 19, 2208233.
- 33.
Wang, W.; Ren, G.; Wang, M.; et al. A novel composite for energy storage devices: Core–shell MnO2/polyindole nanotubes supported on reduced graphene oxides. J. Mater.Sci. Mater. Electron. 2018, 29, 5548–5560.
- 34.
Wang, J.; Gao, X.; Wang, Y.; et al. Robust ring insoluble naphthoquinone derivative cathode with high loading and long cycle life for aqueous zinc organic batteries. Nano Res. Energy 2024, 3, e9120124.
- 35.
Liu, W.; Qiao, F.; Zhao, J. Tuning electrochemical properties of carbon nanofiber electrodes with selenium heteroatoms for flexible zinc ion capacitors. Nano Res. Energy 2024, 3, e9120131.

This work is licensed under a Creative Commons Attribution 4.0 International License.