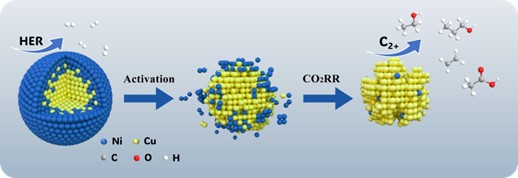
The electrochemical CO2 reduction reaction (CO2RR) has attracted significant attention as a promising strategy for storing intermittent energy in chemical bonds while sustainably producing value-added chemicals and fuels. Copper-based bimetallic catalysts are particularly appealing for CO2RR due to their unique ability to generate multi-carbon products. While substantial effort has been devoted to developing new catalysts, the evolution of bimetallic systems under operational conditions remains underexplored. In this work, we synthesized a series of CuxNi1−x nanoparticles and investigated their structural evolution during CO2RR. Due to the higher oxophilicity of Ni compared to Cu, the particles tend to become Ni-enriched at the surface upon air exposure, promoting the competing hydrogen evolution reaction (HER). At negative activation potentials, cathodic corrosion has been observed in CuxNi1−x nanoparticles, leading to the significant Ni loss and the formation of irregularly shaped Cu nanoparticles with increased defects. This structural evolution, driven by cathodic corrosion, shifts the electrolysis from HER toward CO2 reduction, significantly enhancing the Faradaic efficiency of multi-carbon products (C2+).
- Open Access
- Article
Cathodic Corrosion-Induced Structural Evolution of CuNi Electrocatalysts for Enhanced CO2 Reduction
- Wenjin Sun 1, †,
- Bokki Min 2, †,
- Maoyu Wang 3,
- Xue Han 4,
- Qiang Gao 1,
- Sooyeon Hwang 5,
- Hua Zhou 3,
- Huiyuan Zhu 1, 2, *
Author Information
Received: 22 Oct 2024 | Revised: 25 Nov 2024 | Accepted: 27 Nov 2024 | Published: 04 Dec 2024
Abstract
Graphical Abstract
Keywords
electrochemical CO2 reduction | bimetallic | nanoparticles | cathodic corrosion | structural evolution
References
- 1.Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 reduction: From the electrochemical to photochemical approach. Adv. Sci. 2017, 4, 1700194.
- 2.Zhang, L.; Zhao, Z.J.; Gong, J. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms. Angew. Chem. Int. Ed. 2017, 56, 11326–11353.
- 3.Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What should we make with CO2 and how can we make it? Joule 2018, 2, 825–832.
- 4.Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: Pathways to C2 products. ACS. Catal. 2018, 8, 1490–1499.
- 5.Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119, 7610–7672.
- 6.Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113.
- 7.Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 reduction: A classification problem. Chemphyschem 2017, 18, 3266–3273.
- 8.Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210.
- 9.Zhao, J.; Xue, S.; Barber, J.; Zhou, Y.; Meng, J.; Ke, X. An overview of Cu-based heterogeneous electrocatalysts for CO2 reduction. J. Mater. Chem. A 2020, 8, 4700–4734.
- 10.Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059.
- 11.Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659.
- 12.Zhao, R.; Ding, P.; Wei, P.; Zhang, L.; Liu, Q.; Luo, Y.; Li, T.; Lu, S.; Shi, X.; Gao, S.; et al. Recent progress in electrocatalytic methanation of CO2 at ambient conditions. Adv. Funct. Mater. 2021, 31, 2009449.
- 13.Okatenko, V.; Loiudice, A.; Newton, M.A.; Stoian, D.C.; Blokhina, A.; Chen, A.N.; Rossi, K.; Buonsanti, R. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical CO2 reduction reaction. J. Am. Chem. Soc. 2023, 145, 5370–5383.
- 14.Song, H.; Tan, Y.C.; Kim, B.; Ringe, S.; Oh, J. Tunable product selectivity in electrochemical CO2 reduction on well-mixed Ni-Cu alloys. ACS Appl. Mater. Interfaces 2021, 13, 55272–55280.
- 15.Wu, Z.-Z.; Gao, F.-Y.; Gao, M.-R. Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction. Energy Environ. Sci. 2021, 14, 1121–1139.
- 16.Favaro, M.; Xiao, H.; Cheng, T.; Goddard, W.A., 3rd; Yano, J.; Crumlin, E.J. Subsurface oxide plays a critical role in CO2 activation by Cu(111) surfaces to form chemisorbed CO2, the first step in reduction of CO2. Proc. Natl. Acad. Sci. USA 2017, 114, 6706–6711.
- 17.Gu, Z.; Shen, H.; Chen, Z.; Yang, Y.; Yang, C.; Ji, Y.; Wang, Y.; Zhu, C.; Liu, J.; Li, J.; et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 2021, 5, 429–440.
- 18.Vasileff, A.; Xu, C.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Surface and interface engineering in copper-based bimetallic materials for selective CO2 electroreduction. Chem. 2018, 4, 1809–1831.
- 19.Du, C.; Mills, J.P.; Yohannes, A.G.; Wei, W.; Wang, L.; Lu, S.; Lian, J.X.; Wang, M.; Guo, T.; Wang, X.; et al. Cascade electrocatalysis via AgCu single-atom alloy and Ag nanoparticles in CO2 electroreduction toward multicarbon products. Nat. Commun. 2023, 14, 6142.
- 20.Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948.
- 21.Chen, C.; Li, Y.; Yu, S.; Louisia, S.; Jin, J.; Li, M.; Ross, M.B.; Yang, P. Cu-Ag tandem catalysts for high-rate CO2 electrolysis toward multicarbons. Joule 2020, 4, 1688–1699.
- 22.Iyengar, P.; Kolb, M.J.; Pankhurst, J.R.; Calle-Vallejo, F.; Buonsanti, R. Elucidating the facet-dependent selectivity for CO2 electroreduction to ethanol of Cu–Ag tandem catalysts. ACS Catal. 2021, 11, 4456–4463.
- 23.Clark, E.L.; Hahn, C.; Jaramillo, T.F.; Bell, A.T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 2017, 139, 15848–15857.
- 24.Chang, C.J.; Lin, S.C.; Chen, H.C.; Wang, J.; Zheng, K.J.; Zhu, Y.; Chen, H.M. Dynamic reoxidation/reduction-driven atomic interdiffusion for highly selective CO2 reduction toward methane. J. Am. Chem. Soc. 2020, 142, 12119–12132.
- 25.Huang, J.; Hormann, N.; Oveisi, E.; Loiudice, A.; De Gregorio, G.L.; Andreussi, O.; Marzari, N.; Buonsanti, R. Potential-induced nanoclustering of metallic catalysts during electrochemical CO2 reduction. Nat. Commun. 2018, 9, 3117.
- 26.Lai, W.; Ma, Z.; Zhang, J.; Yuan, Y.; Qiao, Y.; Huang, H. Dynamic evolution of active sites in electrocatalytic CO2 reduction reaction: Fundamental understanding and recent progress. Adv. Funct. Mater. 2022, 32, 2111193.
- 27.Vavra, J.; Ramona, G.P.L.; Dattila, F.; Kormányos, A.; Priamushko, T.; Albertini, P.P.; Loiudice, A.; Cherevko, S.; Lopéz, N.; Buonsanti, R. Solution-based Cu+ transient species mediate the reconstruction of copper electrocatalysts for CO2 reduction. Nat. Catal. 2024, 7, 89–97.
- 28.Lee, S.H.; Lin, J.C.; Farmand, M.; Landers, A.T.; Feaster, J.T.; Aviles Acosta, J.E.; Beeman, J.W.; Ye, Y.; Yano, J.; Mehta, A.; et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ X-ray characterization. J. Am. Chem. Soc. 2021, 143, 588–592.
- 29.Kim, Y.G.; Baricuatro, J.H.; Javier, A.; Gregoire, J.M.; Soriaga, M.P. The evolution of the polycrystalline copper surface, first to Cu(111) and then to Cu(100), at a fixed CO2RR potential: A study by operando EC-STM. Langmuir 2014, 30, 15053–15056.
- 30.Delmo, E.P.; Wang, Y.; Song, Y.; Zhu, S.; Zhang, H.; Xu, H.; Li, T.; Jang, J.; Kwon, Y.; Wang, Y.; et al. In Situ infrared spectroscopic evidence of enhanced electrochemical CO2 reduction and C-C coupling on oxide-derived copper. J. Am. Chem. Soc. 2024, 146, 1935–1945.
- 31.Vavra, J.; Shen, T.H.; Stoian, D.; Tileli, V.; Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem. Int. Ed. 2021, 60, 1347–1354.
- 32.Chen, P.C.; Chen, C.; Yang, Y.; Maulana, A.L.; Jin, J.; Feijoo, J.; Yang, P. Chemical and structural evolution of AgCu catalysts in electrochemical CO2 reduction. J. Am. Chem. Soc. 2023, 145, 10116–10125.
- 33.Jeon, H.S.; Timoshenko, J.; Scholten, F.; Sinev, I.; Herzog, A.; Haase, F.T.; Roldan Cuenya, B. Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction. J. Am. Chem. Soc. 2019, 141, 19879–19887.
- 34.Gao, Q.; Ju, Y.M.; An, D.; Gao, M.R.; Cui, C.H.; Liu, J.W.; Cong, H.P.; Yu, S.H. Shape-controlled synthesis of monodisperse PdCu nanocubes and their electrocatalytic properties. ChemSusChem 2013, 6, 1878–1882.
- 35.Wang, Y.; Xu, A.; Wang, Z.; Huang, L.; Li, J.; Li, F.; Wicks, J.; Luo, M.; Nam, D.H.; Tan, C.S.; et al. Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708.
- 36.Liu, S.; Li, Y.; Wang, D.; Xi, S.; Xu, H.; Wang, Y.; Li, X.; Zang, W.; Liu, W.; Su, M.; et al. Alkali cation-induced cathodic corrosion in Cu electrocatalysts. Nat. Commun. 2024, 15, 5080.
- 37.McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010; pp. 111–112.
- 38.Hersbach, T.J.P.; Koper, M.T.M. Cathodic corrosion: 21st century insights into a 19th century phenomenon. Curr. Opin. Electrochem. 2021, 26, 100653.
- 39.Nong, H.N.; Reier, T.; Oh, H.-S.; Gliech, M.; Paciok, P.; Vu, T.H.T.; Teschner, D.; Heggen, M.; Petkov, V.; Schlögl, R.; et al. A unique oxygen ligand environment facilitates water oxidation in hole-doped IrNiOx core–shell electrocatalysts. Nat. Catal. 2018, 1, 841–851.
- 40.Lin, S.C.; Chang, C.C.; Chiu, S.Y.; Pai, H.T.; Liao, T.Y.; Hsu, C.S.; Chiang, W.H.; Tsai, M.K.; Chen, H.M. Operando time-resolved X-ray absorption spectroscopy reveals the chemical nature enabling highly selective CO2 reduction. Nat. Commun. 2020, 11, 3525.
- 41.Pan, H.; Barile, C.J. Bifunctional nickel and copper electrocatalysts for CO2 reduction and the oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 1741–1748.
How to Cite
Sun, W.; Min, B.; Wang, M.; Han, X.; Gao, Q.; Hwang, S.; Zhou, H.; Zhu, H. Cathodic Corrosion-Induced Structural Evolution of CuNi Electrocatalysts for Enhanced CO2 Reduction. Materials and Interfaces 2024, 1 (1), 79–88. https://doi.org/10.53941/mi.2024.100007.
RIS
BibTex
Copyright & License

Copyright (c) 2024 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


