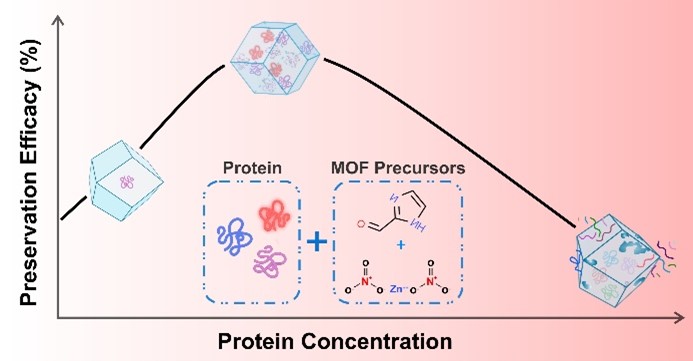
Metal-organic frameworks (MOFs) have emerged as attractive bioencapsulants for preserving the structure and function of various biomolecules against harsh environmental conditions. However, the effect of the loading density of the biomolecules on the structure, physical properties, and biopreservation efficacy of MOF crystals remains elusive. We investigated the structure and properties of zeolitic imidazolate framework (ZIF)-90 crystals as a function of the loading density of a model protein, bovine/human serum albumin (BSA/HSA). We show that the total protein concentration in the MOF growth reaction solution significantly affects the morphology, degree of crystallinity, and biopreservation efficacy of the MOF crystals. The structure integrity and immunologic functionality of albumin remained well-preserved within an optimal protein concentration range of 0.1–1 mg/mL. The proposed optimal range of biomolecule concentration during in situ MOF growth is critical for guiding future research and design endeavors within the rapidly evolving field of MOF-biomedical applications, offering exciting possibilities for biopreservation, drug delivery, and diagnostics.
- Open Access
- Article
Effect of Protein Loading Density on the Structure and Biopreservation Efficacy of Metal-Organic Frameworks
- Yixuan Wang 1, 2,
- Sirimuvva Tadepalli 3, †,
- Harsh Baldi 1, 2,
- Jeremiah J. Morrissey 1, 4,
- Srikanth Singamaneni 1, 2, 4, *
Author Information
Received: 09 Sep 2024 | Revised: 31 Oct 2024 | Accepted: 05 Nov 2024 | Published: 20 Nov 2024
Abstract
Graphical Abstract
References
- 1.Schrohl, A.S.; Würtz, S.; Kohn, E.; Banks, R.E.; Nielsen, H.J.; Sweep, F.C.; Brünner, N. Banking of biological fluids for studies of disease-associated protein biomarkers. Mol. Cell. Proteom. 2008, 7, 2061–2066. https://doi.org/10.1074/mcp.R800010-MCP200.
- 2.Chaigneau, C.; Cabioch, T.; Beaumont, K.; Betsou, F. Serum biobank certification and the establishment of quality controls for biological fluids: Examples of serum biomarker stability after temperature variation. Clin. Chem. Lab. Med. 2007, 45, 1390–1395. https://doi.org/10.1515/cclm.2007.160.
- 3.Luan, J.; Seth, A.; Gupta, R.; Wang, Z.; Rathi, P.; Cao, S.; Gholami Derami, H.; Tang, R.; Xu, B.; Achilefu, S.; et al. Ultrabright fluorescent nanoscale labels for the femtomolar detection of analytes with standard bioassays. Nat. Biomed. Eng. 2020, 4, 518–530. https://doi.org/10.1038/s41551-020-0547-4.
- 4.Livesey, J.H.; Ellis, M.J.; Evans, M.J. Pre-analytical requirements. Clin. Biochem. Rev. 2008, 29, S11–S15.
- 5.Evans, M.J.; Livesey, J.H.; Ellis, M.J.; Yandle, T.G. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin. Biochem. 2001, 34, 107–112. https://doi.org/10.1016/s0009-9120(01)00196-5.
- 6.Zhang, J.; Pritchard, E.; Hu, X.; Valentin, T.; Panilaitis, B.; Omenetto, F.G.; Kaplan, D.L. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. 2012, 109, 11981–11986, doi:doi:10.1073/pnas.1206210109.
- 7.Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 1–8.
- 8.Wijesundara, Y.H.; Herbert, F.C.; Trashi, O.; Trashi, I.; Brohlin, O.R.; Kumari, S.; Howlett, T.; Benjamin, C.E.; Shahrivarkevishahi, A.; Diwakara, S.D.; et al. Carrier gas triggered controlled biolistic delivery of DNA and protein therapeutics from metal–organic frameworks. Chem. Sci. 2022, 13, 13803–13814. https://doi.org/10.1039/d2sc04982a.
- 9.Wang, C.; Sun, H.; Luan, J.; Jiang, Q.; Tadepalli, S.; Morrissey, J.J.; Kharasch, E.D.; Singamaneni, S. Metal–Organic Framework Encapsulation for Biospecimen Preservation. Chem. Mater. 2018, 30, 1291–1300. https://doi.org/10.1021/acs.chemmater.7b04713.
- 10.Wang, Y.; Morrissey, J.J.; Gupta, P.; Chauhan, P.; Pachynski, R.K.; Harris, P.K.; Chaudhuri, A.; Singamaneni, S. Preservation of Proteins in Human Plasma through Metal-Organic Framework Encapsulation. ACS Appl. Mater. Interfaces 2023, 15, 18598–18607. https://doi.org/10.1021/acsami.2c21192.
- 11.Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, C.J. Metal-Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. https://doi.org/10.1021/acs.chemrev.0c01029.
- 12.Wang, A.; Walden, M.; Ettlinger, R.; Kiessling, F.; Gassensmith, J.J.; Lammers, T.; Wuttke, S.; Peña, Q. Biomedical Metal–Organic Framework Materials: Perspectives and Challenges. Adv. Funct. Mater. 2023, 34, 2308589. https://doi.org/10.1002/adfm.202308589.
- 13.Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444.
- 14.Morris, W.; Doonan, C.J.; Furukawa, H.; Banerjee, R.; Yaghi, O.M. Crystals as Molecules: Postsynthesis Covalent Functionalization of Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2008, 130, 12626–12627. https://doi.org/10.1021/ja805222x.
- 15.Gao, S.; Hou, J.; Deng, Z.; Wang, T.; Beyer, S.; Buzanich, A.G.; Richardson, J.J.; Rawal, A.; Seidel, R.; Zulkifli, M.Y.; et al. Improving the Acidic Stability of Zeolitic Imidazolate Frameworks by Biofunctional Molecules. Chem 2019, 5, 1597–1608. https://doi.org/10.1016/j.chempr.2019.03.025.
- 16.Wang, C.; Sudlow, G.; Wang, Z.; Cao, S.; Jiang, Q.; Neiner, A.; Morrissey, J.J.; Kharasch, E.D.; Achilefu, S.; Singamaneni, S. Metal-Organic Framework Encapsulation Preserves the Bioactivity of Protein Therapeutics. Adv. Healthc. Mater. 2018, 7, e1800950. https://doi.org/10.1002/adhm.201800950.
- 17.Kang, L.; Smith, S.; Wang, C. Metal–Organic Framework Preserves the Biorecognition of Antibodies on Nanoscale Surfaces Validated by Single-Molecule Force Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 3011–3020. https://doi.org/10.1021/acsami.9b19551.
- 18.Liang, K.; Coghlan, C.J.; Bell, S.G.; Doonan, C.; Falcaro, P. Enzyme encapsulation in zeolitic imidazolate frameworks: A comparison between controlled co-precipitation and biomimetic mineralisation. Chem. Commun. 2016, 52, 473–476.
- 19.Liang, W.; Xu, H.; Carraro, F.; Maddigan, N.K.; Li, Q.; Bell, S.G.; Huang, D.M.; Tarzia, A.; Solomon, M.B.; Amenitsch, H.; et al. Enhanced Activity of Enzymes Encapsulated in Hydrophilic Metal–Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 2348–2355. https://doi.org/10.1021/jacs.8b10302.
- 20.Luzuriaga, M.A.; Herbert, F.C.; Brohlin, O.R.; Gadhvi, J.; Howlett, T.; Shahrivarkevishahi, A.; Wijesundara, Y.H.; Venkitapathi, S.; Veera, K.; Ehrman, R. Metal–Organic Framework Encapsulated Whole-Cell Vaccines Enhance Humoral Immunity against Bacterial Infection. ACS Nano 2021, 15, 17426–17438.
- 21.Luzuriaga, M.A.; Welch, R.P.; Dharmarwardana, M.; Benjamin, C.E.; Li, S.; Shahrivarkevishahi, A.; Popal, S.; Tuong, L.H.; Creswell, C.T.; Gassensmith, J.J. Enhanced stability and controlled delivery of MOF-encapsulated vaccines and their immunogenic response in vivo. ACS Appl. Mater. Interfaces 2019, 11, 9740–9746.
- 22.Ehrman, R.N.; Brohlin, O.R.; Wijesundara, Y.H.; Kumari, S.; Trashi, O.; Howlett, T.S.; Trashi, I.; Herbert, F.C.; Raja, A.; Koirala, S.; et al. A scalable synthesis of adjuvanting antigen depots based on metal–organic frameworks. Chem. Sci. 2024, 15, 2731–2744. https://doi.org/10.1039/d3sc06734c.
- 23.Liang, K.; Richardson, J.J.; Cui, J.; Caruso, F.; Doonan, C.J.; Falcaro, P. Metal–organic framework coatings as cytoprotective exoskeletons for living cells. Adv. Mater. 2016, 28, 7910–7914.
- 24.Wang, C.; Tadepalli, S.; Luan, J.; Liu, K.K.; Morrissey, J.J.; Kharasch, E.D.; Naik, R.R.; Singamaneni, S. Metal-Organic Framework as a Protective Coating for Biodiagnostic Chips. Adv. Mater. 2017, 29, 1604433. https://doi.org/10.1002/adma.201604433.
- 25.Velásquez-Hernández, M.d.J.; Linares-Moreau, M.; Astria, E.; Carraro, F.; Alyami, M.Z.; Khashab, N.M.; Sumby, C.J.; Doonan, C.J.; Falcaro, P. Towards applications of bioentities@MOFs in biomedicine. Coord. Chem. Rev. 2021, 429, 213651. https://doi.org/10.1016/j.ccr.2020.213651.
- 26.Zhang, H.; Lv, Y.; Tan, T.; van der Spoel, D. Atomistic Simulation of Protein Encapsulation in Metal-Organic Frameworks. J. Phys. Chem. B 2016, 120, 477–484. https://doi.org/10.1021/acs.jpcb.5b10437.
- 27.Wang, Y.; Wang, Z.; Gupta, P.; Morrissey, J.J.; Naik, R.R.; Singamaneni, S. Enhancing the Stability of COVID-19 Serological Assay through Metal-Organic Framework Encapsulation. Adv. Healthc. Mater. 2021, 10, e2100410. https://doi.org/10.1002/adhm.202100410.
- 28.Ogata, A.F.; Rakowski, A.M.; Carpenter, B.P.; Fishman, D.A.; Merham, J.G.; Hurst, P.J.; Patterson, J.P. Direct Observation of Amorphous Precursor Phases in the Nucleation of Protein-Metal-Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 1433–1442. https://doi.org/10.1021/jacs.9b11371.
- 29.Carraro, F.; Williams, J.D.; Linares-Moreau, M.; Parise, C.; Liang, W.; Amenitsch, H.; Doonan, C.; Kappe, C.O.; Falcaro, P. Continuous-Flow Synthesis of ZIF-8 Biocomposites with Tunable Particle Size. Angew. Chem. Int. Ed. Engl. 2020, 59, 8123–8127. https://doi.org/10.1002/anie.202000678.
- 30.Troyano, J.; Carne-Sanchez, A.; Avci, C.; Imaz, I.; Maspoch, D. Colloidal metal-organic framework particles: The pioneering case of ZIF-8. Chem. Soc. Rev. 2019, 48, 5534–5546. https://doi.org/10.1039/c9cs00472f.
- 31.Carraro, F.; Velásquez-Hernández, M.d.J.; Astria, E.; Liang, W.; Twight, L.; Parise, C.; Ge, M.; Huang, Z.; Ricco, R.; Zou, X.; et al. Phase dependent encapsulation and release profile of ZIF-based biocomposites. Chem. Sci. 2020, 11, 3397–3404. https://doi.org/10.1039/C9SC05433B.
- 32.Linares-Moreau, M.; Brandner, L.A.; Velasquez-Hernandez, M.J.; Fonseca, J.; Benseghir, Y.; Chin, J.M.; Maspoch, D.; Doonan, C.; Falcaro, P. Fabrication of Oriented Polycrystalline MOF Superstructures. Adv. Mater. 2024, 36, e2309645. https://doi.org/10.1002/adma.202309645.
- 33.Lo, Y.; Lam, C.H.; Chang, C.-W.; Yang, A.-C.; Kang, D.-Y. Polymorphism/pseudopolymorphism of metal–organic frameworks composed of zinc(ii) and 2-methylimidazole: Synthesis, stability, and application in gas storage. RSC Adv. 2016, 6, 89148–89156. https://doi.org/10.1039/c6ra19437k.
- 34.Herbert, F.C.; Abeyrathna, S.S.; Abeyrathna, N.S.; Wijesundara, Y.H.; Brohlin, O.R.; Carraro, F.; Amenitsch, H.; Falcaro, P.; Luzuriaga, M.A.; Durand-Silva, A.; et al. Stabilization of supramolecular membrane protein-lipid bilayer assemblies through immobilization in a crystalline exoskeleton. Nat. Commun. 2021, 12, 2202. https://doi.org/10.1038/s41467-021-22285-y.
- 35.Tocco, D.; Chelazzi, D.; Mastrangelo, R.; Casini, A.; Salis, A.; Fratini, E.; Baglioni, P. Conformational changes and location of BSA upon immobilization on zeolitic imidazolate frameworks. J. Colloid. Interface Sci. 2023, 641, 685–694. https://doi.org/10.1016/j.jcis.2023.03.107.
- 36.Bakhshandeh, A.; Ardestani, F.; Ghorbani, H.R.; Darvish Ganji, M. Structural and molecular properties of complexes of biomolecules and metal-organic frameworks: Dispersion-corrected DFT treatment. J. Mol. Model. 2022, 28, 32. https://doi.org/10.1007/s00894-021-04947-2.
- 37.Mittal, A.; Gandhi, S.; Roy, I. Mechanistic interaction studies of synthesized ZIF-8 nanoparticles with bovine serum albumin using spectroscopic and molecular docking approaches. Sci. Rep. 2022, 12, 10331. https://doi.org/10.1038/s41598-022-14630-y.
- 38.Xu, Z.; Zhang, J.; Pan, T.; Li, H.; Huo, F.; Zheng, B.; Zhang, W. Encapsulation of Hydrophobic Guests within Metal–Organic Framework Capsules for Regulating Host–Guest Interaction. Chem. Mater. 2020, 32, 3553–3560. https://doi.org/10.1021/acs.chemmater.0c00684.
- 39.Marsh, C.; Shearer, G.C.; Knight, B.T.; Paul-Taylor, J.; Burrows, A.D. Supramolecular aspects of biomolecule interactions in metal–organic frameworks. Coord. Chem. Rev. 2021, 439, 213928. https://doi.org/10.1016/j.ccr.2021.213928.
- 40.Chen, G.; Huang, S.; Kou, X.; Zhu, F.; Ouyang, G. Embedding Functional Biomacromolecules within Peptide-Directed Metal-Organic Framework (MOF) Nanoarchitectures Enables Activity Enhancement. Angew. Chem. Int. Ed. Engl. 2020, 59, 13947–13954. https://doi.org/10.1002/anie.202005529.
- 41.Akhundzadeh Tezerjani, A.; Halladj, R.; Askari, S. Different view of solvent effect on the synthesis methods of zeolitic imidazolate framework-8 to tuning the crystal structure and properties. RSC Adv. 2021, 11, 19914–19923. https://doi.org/10.1039/d1ra02856a.
- 42.Schmidt, M.P.; Martinez, C.E. Kinetic and Conformational Insights of Protein Adsorption onto Montmorillonite Revealed Using in Situ ATR-FTIR/2D-COS. Langmuir 2016, 32, 7719–7729. https://doi.org/10.1021/acs.langmuir.6b00786.
- 43.Barreto, M.S.C.; Elzinga, E.J.; Alleoni, L.R.F. The molecular insights into protein adsorption on hematite surface disclosed by in-situ ATR-FTIR/2D-COS study. Sci. Rep. 2020, 10, 13441. https://doi.org/10.1038/s41598-020-70201-z.
- 44.Carpenter, B.P.; Talosig, A.R.; Mulvey, J.T.; Merham, J.G.; Esquivel, J.; Rose, B.; Ogata, A.F.; Fishman, D.A.; Patterson, J.P. Role of Molecular Modification and Protein Folding in the Nucleation and Growth of Protein-Metal-Organic Frameworks. Chem. Mater. 2022, 34, 8336–8344. https://doi.org/10.1021/acs.chemmater.2c01903.
- 45.Murty, R.; Bera, M.K.; Walton, I.M.; Whetzel, C.; Prausnitz, M.R.; Walton, K.S. Interrogating Encapsulated Protein Structure within Metal-Organic Frameworks at Elevated Temperature. J. Am. Chem. Soc. 2023, 145, 7323–7330. https://doi.org/10.1021/jacs.2c13525.
- 46.Lu, R.; Li, W.W.; Katzir, A.; Raichlin, Y.; Yu, H.Q.; Mizaikoff, B. Probing the secondary structure of bovine serum albumin during heat-induced denaturation using mid-infrared fiberoptic sensors. Analyst 2015, 140, 765–770. https://doi.org/10.1039/c4an01495b.
- 47.Ranjan, S.; Dasgupta, N.; Srivastava, P.; Ramalingam, C. A spectroscopic study on interaction between bovine serum albumin and titanium dioxide nanoparticle synthesized from microwave-assisted hybrid chemical approach. J. Photochem. Photobiol. B 2016, 161, 472–481. https://doi.org/10.1016/j.jphotobiol.2016.06.015.
- 48.Abrosimova, K.V.; Shulenina, O.V.; Paston, S.V. FTIR study of secondary structure of bovine serum albumin and ovalbumin. J. Phys. Conf. Ser. 2016, 769, 012016. https://doi.org/10.1088/1742-6596/769/1/012016.
- 49.Ketrat, S.; Japrung, D.; Pongprayoon, P. Exploring how structural and dynamic properties of bovine and canine serum albumins differ from human serum albumin. J. Mol. Graph. Model. 2020, 98, 107601. https://doi.org/10.1016/j.jmgm.2020.107601.
How to Cite
Wang, Y.; Tadepalli, S.; Baldi, H.; Morrissey, J. J.; Singamaneni, S. Effect of Protein Loading Density on the Structure and Biopreservation Efficacy of Metal-Organic Frameworks. Materials and Interfaces 2024, 1 (1), 47–57. https://doi.org/10.53941/mi.2024.100003.
RIS
BibTex
Copyright & License

Copyright (c) 2024 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


