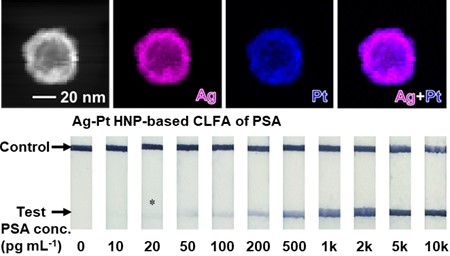
Colorimetric lateral flow assay (CLFA) has been a widely recognized point-of-care testing technology over the past few decades. Driven by the increasing demand in various biomedical applications, it is urgently needed to develop CLFAs with high sensitivities and low costs. In this work, we report a type of CLFA that relies on unique colorimetric labels—silver-platinum hollow nanoparticles (Ag-Pt HNPs). The Ag-Pt HNPs possess intrinsic enzyme-like catalytic activities, providing the Ag-Pt HNP-based CLFA with strong color signal and thus a high sensitivity. Meanwhile, the Ag-Pt HNPs have hollow interiors and are mainly composed of less expensive silver, making the Ag-Pt HNP-based CLFA cost-effective. Using prostate-specific antigen (PSA) as a model disease biomarker, the Ag-Pt HNP-based CLFA achieved a high sensitivity with a detection limit at the low picogram-per-milliliter level. Potential application of the CLFA in clinical diagnosis was demonstrated by detecting PSA from human serum samples.
- Open Access
- Article
Silver-Platinum Hollow Nanoparticles as Labels for Colorimetric Lateral Flow Assay
Author Information
Received: 23 Sep 2024 | Revised: 07 Nov 2024 | Accepted: 12 Nov 2024 | Published: 18 Nov 2024
Abstract
Graphical Abstract
Keywords
hollow nanoparticles | lateral flow assay | catalysis | biomarker | detection
References
- 1.Budd, J.; Miller, B.S.; Weckman, N.E.; Cherkaoui, D.; Huang, D.; Decruz, A.T.; Fongwen, N.; Hanet, G.-R.; Broto, M.; Estcourt, C.S.; et al. Lateral Flow Test Engineering and Lessons Learned from COVID-19. Nat. Rev. Bioeng. 2023, 1, 13–31.
- 2.Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611.
- 3.Jiang, N.; Tansukawat, N.D.; Gonzalez-Macia, L.; Ates, H.C.; Dincer, C.; Guder, F.; Tasoglu, S.; Yetisen, A.K. Low-Cost Optical Assays for Point-of-Care Diagnosis in Resource-Limited Settings. ACS Sens. 2021, 6, 2108–2124.
- 4.Khlebtsov, B.N.; Tumskiy, R.S.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Quantifying the Numbers of Gold Nanoparticles in the Test Zone of Lateral Flow Immunoassay Strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028.
- 5.Dzhagan, V.; Kapush, O.; Plokhovska, S.; Buziashvili, A.; Pirko, Y.; Yeshchenko, O.; Yukhymchuk, V.; Yemets, A.; Zahn, D.R.T. Plasmonic Colloidal Au Nanoparticles in DMSO: A Facile Synthesis and Characterisation. RSC Adv. 2022, 12, 21591–21599.
- 6.Yuan, Z.; Hu, C.C.; Chang, H.T.; Lu, C. Gold Nanoparticles as Sensitive Optical Probes. Analyst 2016, 141, 1611–1626.
- 7.Cui, X.; Huang, Y.; Wang, J.; Zhang, L.; Rong, Y.; Lai, W.; Chen, T. A Remarkable Sensitivity Enhancement in a Gold Nanoparticle-Based Lateral Flow Immunoassay for the Detection of Escherichia coli O157:H7. RSC Adv. 2015, 5, 45092–45097.
- 8.Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-Decorated Gold Nanoparticles with Dual Functionalities for Ultrasensitive Colorimetric In Vitro Diagnostics. Nano Lett. 2017, 17, 5572–5579.
- 9.Zhan, L.; Guo, S.Z.; Song, F.; Gong, Y.; Xu, F.; Boulware, D.R.; McAlpine, M.C.; Chan, W.C.W.; Bischof, J.C. The Role of Nanoparticle Design in Determining Analytical Performance of Lateral Flow Immunoassays. Nano Lett. 2017, 17, 7207–7212.
- 10.Ji, Y.; Ren, M.; Li, Y.; Huang, Z.; Shu, M.; Yang, H.; Xiong, Y.; Xu, Y. Detection of Aflatoxin B-1 with Immunochromatographic Test Strips: Enhanced Signal Sensitivity Using Gold Nanoflowers. Talanta 2015, 142, 206–212.
- 11.Ge, C.; Wu, R.; Chong, Y.; Fang, G.; Jiang, X.; Pan, Y.; Chen, C.; Yin, J.J. Synthesis of Pt Hollow Nanodendrites with Enhanced Peroxidase-like Activity against Bacterial Infections: Implication for Wound Healing. Adv. Funct. Mater. 2018, 28, 1801484.
- 12.Renzi, E.; Piper, A.; Nastri, F.; Merkoçi, A.; Lombardi, A. An Artificial Miniaturized Peroxidase for Signal Amplification in Lateral Flow Immunoassays. Small 2023, 19, 2207949.
- 13.Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 2018, 12, 279–288.
- 14.Wei, Z.; Luciano, K.; Xia, X. Catalytic Gold-Iridium Nanoparticles as Labels for Sensitive Colorimetric Lateral Flow Assay. ACS Nano 2022, 16, 21609–21617.
- 15.Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F.; et al. Standardized Assays for Determining the Catalytic Activity and Kinetics of Peroxidase-like Nanozymes. Nat. Protoc. 2018, 13, 1506–1520.
- 16.Gao, W.; Eastwood, H.; Xia, X. Peroxidase Mimics of Platinum-Group Metals for In Vitro Diagnostics: Opportunities and Challenges. J. Mater. Chem. B 2023, 11, 8404–8410.
- 17.Wei, Z.; Xi, Z.; Vlasov, S.; Ayala, J.; Xia, X. Nanocrystals of Platinum-Group Metals as Peroxidase Mimics for In Vitro Diagnostics. Chem. Commun. 2020, 56, 14962–14975.
- 18.Naseri, M.; Ziora, Z.M.; Simon, G.P.; Batchelor, W. ASSURED-Compliant Point-of-Care Diagnostics for the Detection of Human Viral Infections. Rev. Med. Virol. 2022, 32, e2263.
- 19.Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846.
- 20.He, M.Q.; Ai, Y.; Hu, W.; Guan, L.; Ding, M.; Liang, Q. Recent Advances of Seed-Mediated Growth of Metal Nanoparticles: From Growth to Applications. Adv. Mater. 2023, 35, 2211915.
- 21.Gao, Z.; Ye, H.; Wang, Q.; Kim, M.J.; Tang, D.; Xi, Z.; Wei, Z.; Shao, S.; Xia, X. Template Regeneration in Galvanic Replacement: A Route to Highly Diverse Hollow Nanostructures. ACS Nano 2020, 14, 791–801.
- 22.Gao, Z.; Shao, S.; Gao, W.; Tang, D.; Tang, D.; Zou, S.; Kim, M.J.; Xia, X. Morphology-Invariant Metallic Nanoparticles with Tunable Plasmonic Properties. ACS Nano 2021, 15, 2428–2438.
- 23.Xia, X.; Zhang, J.; Lu, N.; Kim, M.J.; Ghale, K.; Xu, Y.; McKenzie, E.; Liu, J.; Ye, H. Pd-Ir Core-Shell Nanocubes: A Type of Highly Efficient and Versatile Peroxidase Mimic. ACS Nano 2015, 9, 9994–10004.
- 24.Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583.
- 25.Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666.
- 26.Biby, A.; Crawford, H.; Xia, X. Platinum-Group Metal Nanoparticles as Peroxidase Mimics: Implications for Biosensing. ACS Appl. Nano Mater. 2022, 5, 17622–17631.
- 27.Lin, H.; Liu, Y.; Huo, J.; Zhang, A.; Pan, Y.; Bai, H.; Jiao, Z.; Fang, T.; Wang, X.; Cai, Y.; et al. Modified Enzyme-Linked Immunosorbent Assay Strategy Using Graphene Oxide Sheets and Gold Nanoparticles Functionalized with Different Antibody Types. Anal. Chem. 2013, 85, 6228–6232.
- 28.Glomm, W.R.; Halskau, Ø.; Hanneseth, A.-M.D.; Volden, S. Adsorption Behavior of Acidic and Basic Proteins onto Citrate-Coated Au Surfaces Correlated to Their Native Fold, Stability, and pI. J. Phys. Chem. B 2007, 111, 14329–14345.
- 29.Ye, H.; Xia, X. Enhancing the Sensitivity of Colorimetric Lateral Flow Assay (CLFA) through Signal Amplification Techniques. J. Mater. Chem. B 2018, 6, 7102–7111.
- 30.Josephy, P.D.; Eling, T.; Mason, R.P. The Horseradish Peroxidase-Catalyzed Oxidation of 3,5,3′,5′-tetramethylbenzidine. Free Radical and Charge-Transfer Complex Intermediates. J. Biol. Chem. 1982, 257, 3669–3675.
- 31.Gao, W.; Sun, X.; Yishay, T.; Wei, Z.; Zhu, X.; Kim, M.J.; Xia, X. Iridium Nanoparticles as Highly Effective Peroxidase Mimics: Synthesis, Characterization, and Application in Biosensing. ChemNanoMat 2024, 10, e202300589.
- 32.Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction Coefficient of Gold Nanoparticles with Different Sizes and Different Capping Ligands. Colloids Surf. B Biointerfaces 2007, 58, 3–7.
- 33.Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.E.; Freiha, F.S.; Redwine, E. Prostate-Specific Antigen as a Serum Marker for Adenocarcinoma of the Prostate. N. Engl. J. Med. 1987, 317, 909–916.
- 34.Palladino, P.; Torrini, F.; Scarano, S.; Minunni, M. 3,3′,5,5′-tetramethylbenzidine as Multi-Colorimetric Indicator of Chlorine in Water in Line with Health Guideline Values. Anal. Bioanal. Chem. 2020, 412, 7861–7869.
- 35.Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52.
- 36.Armbruster, D.A.; Tillman, M.D.; Hubbs, L.M. Limit of Detection (LQD)/limit of Quantitation (LOQ): Comparison of the Empirical and the Statistical Methods Exemplified with GC-MS Assays of Abused Drugs. Clin. Chem. 1994, 40, 1233–1238.
- 37.Zerva, L.; Bourantas, K.; Mitka, S.; Kansouzidou, A.; Legakis, N.J. Serum is the Preferred Clinical Specimen for Diagnosis of Human Brucellosis by PCR. J. Clin. Microbiol. 2001, 39, 1661–1664.
- 38.Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X. Characterization of MicroRNAs in Serum: A Novel Class of Biomarkers for Diagnosis of Cancer and Other Diseases. Cell Res., 2008, 18, 997–1006.
- 39.Wegner, K.D.; Jin, Z.; Linden, S.; Jennings, T.L.; Hildebrandt, N. Quantum-Dot-Based Förster Resonance Energy Transfer Immunoassay for Sensitive Clinical Diagnostics of Low-Volume Serum Samples. ACS Nano 2013, 7, 7411–7419.
- 40.Harris, D.C. Quantitative Chemical Analysis, 9th Ed.; W.H. Freeman & Company: New York, NY, USA, 2016.
How to Cite
Zhou, J.; Shao, S.; Wei, Z.; Xia, X. Silver-Platinum Hollow Nanoparticles as Labels for Colorimetric Lateral Flow Assay. Materials and Interfaces 2024, 1 (1), 58–67. https://doi.org/10.53941/mi.2024.100002.
RIS
BibTex
Copyright & License

Copyright (c) 2024 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


