The ability to synthesize nanoparticles of desired shape, size and composition relies heavily on our understanding on how to finely control various factors influencing the formation, such as the kinetics of growth. Fundamental study on the nucleation and growth of nanoparticles found itself at the forefront with the application of liquid-phase transmission electron microscopy (LTEM) in the investigation of dynamic growth and assembly processes. Since early study using LTEM to observe and quantify the nucleation and growth of single colloidal platinum nanoparticles, several theoretical models have been developed. More complex mode of formation was also revealed based on a hybrid growth process of gold on platinum icosahedral nanoparticles to form core-shell structures. These studies have been carried out by focusing on single or a small number of nanoparticles. Herewith, we present a study on the establishment of an analytical method to quantify the particle formation using in situ LTEM technique. This approach is based on the analysis of median particle size and focused on main events accounted for the formation of nanoparticles at a given time. We found that unlike the cases for single particle analysis, the observed formation rate could not be explained by any single formation mode, such as diffusion- and/or reaction-controlled growth described by the Liftshitz-Slyosov-Wagner theory or formation through coalescence as described by the Smoluchowski aggregative kinetics. A global fit was used to describe the entire formation of nanoparticles in an ensemble.
- Open Access
- Article
Modelling the Growth and Aggregation of Gold Nanoparticles Using Liquid-Phase Transmission Electron Microscopy
- Thao Ngo,
- Siying Yu,
- Hong Yang *
Author Information
Received: 02 Aug 2024 | Revised: 05 Jun 2025 | Accepted: 08 Jun 2025 | Published: 18 Jun 2025
Abstract
Graphical Abstract
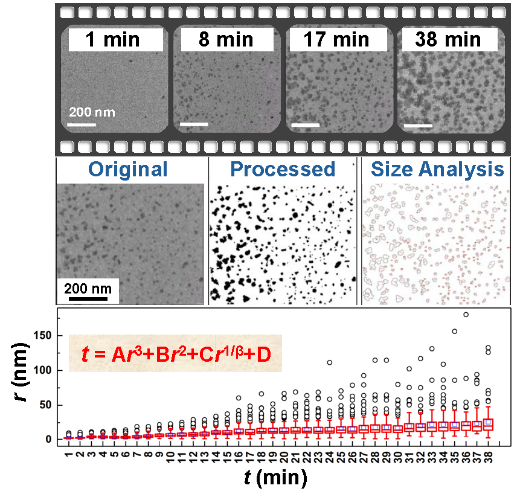
Keywords
LTEM | modelling | nanoparticle | growth | aggregation
References
- 1.Lu, J.; Wu, W.; Colombari, F.M.; Jawaid, A.; Seymour, B.; Whisnant, K.; Zhong, X.; Choi, W.; Chalmpes, N.; Lahann, J.; et al. Nano-Achiral Complex Composites for Extreme Polarization Optics. Nature 2024, 630, 860–865.
- 2.Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The Surface Science of Nanocrystals. Nat. Mater. 2016, 15, 141–153.
- 3.Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488.
- 4.Murphy, C.J.; Vartanian, A.M.; Geiger, F.M.; Hamers, R.J.; Pedersen, J.; Cui, Q.; Haynes, C.L.; Carlson, E.E.; Hernandez, R.; Klaper, R.D.; et al. Biological Responses to Engineered Nanomaterials: Needs for the Next Decade. ACS Cent. Sci. 2015, 1, 117–123.
- 5.Wu, J.; Yang, H. Platinum-Based Oxygen Reduction Electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857.
- 6.Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389.
- 7.Zhou, L.; Huang, Q.; Xia, Y. Plasmon-Induced Hot Electrons in Nanostructured Materials: Generation, Collection, and Application to Photochemistry. Chem. Rev. 2024, 124, 8597–8619.
- 8.Yu, S.; Yang, H. Design Principles for the Synthesis of Platinum–Cobalt Intermetallic Nanoparticles for Electrocatalytic Applications. Chem. Commun. 2023, 59, 4852–4871.
- 9.Peng, Z.; You, H.; Yang, H. Composition-Dependent Formation of Platinum Silver Nanowires. ACS Nano 2010, 4, 1501–1510.
- 10.Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem., Int. Ed. 2007, 46, 4630–4660.
- 11.Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 60–103.
- 12.Yin, X.; Shi, M.; Wu, J.; Pan, Y.-T.; Gray, D.L.; Bertke, J.A.; Yang, H. Quantitative Analysis of Different Formation Modes of Pt Nanocrystals Controlled by Ligand Chemistry. Nano Lett. 2017, 17, 6146–6150.
- 13.Yu, S.; Zhang, C.; Yang, H. Two-Dimensional Metal Nanostructures: From Theoretical Understanding to Experiment. Chem. Rev. 2023, 123, 3443–3492.
- 14.Drake, G.A.; Keating, L.P.; Shim, M. Design Principles of Colloidal Nanorod Heterostructures. Chem. Rev. 2023, 123, 3761–3789.
- 15.Grogan, J.M.; Rotkina, L.; Bau, H.H. In-Situ Liquid-Cell Electron Microscopy of Colloid Aggregation and Growth Dynamics. Phys. Rev. E 2011, 83, 061405.
- 16.Liao, H.G.; Cui, L.; Whitelam, S.; Zheng, H. Real-Time Imaging of Pt3Fe Nanorod Growth in Solution. Science 2012, 336, 1011–1014.
- 17.Wu, J.; Gao, W.; Yang, H.; Zuo, J.-M. Dissolution Kinetics of Oxidative Etching of Cubic and Icosahedral Platinum Nanoparticles Revealed by in situ Liquid Transmission Electron Microscope. ACS Nano 2017, 11, 1696–1703.
- 18.Lyu, Z.; Yao, L.; Chen, W.; Kalutantirige, F.C.; Chen, Q. Electron Microscopy Studies of Soft Nanomaterials. Chem. Rev. 2023, 123, 4051–4145.
- 19.Kim, B.H.; Yang, J.; Lee, D.; Choi, B.K.; Hyeon, T.; Park, J. Liquid-Phase Transmission Electron Microscopy for Studying Colloidal Inorganic Nanoparticles. Adv. Mater. 2018, 30, 1703316.
- 20.Kim, B.H.; Heo, J.; Kim, S.; Reboul, C.F.; Chun, H.; Kang, D.; Bae, H.; Hyun, H.; Lim, J.; Lee, H.; et al. Critical Differences in 3D Atomic Structure of Individual Ligand-Protected Nanocrystals in Solution. Science 2020, 368, 60–67.
- 21.Ngo, T.; Yang, H. Toward Ending the Guessing Game: Study of the Formation of Nanostructures Using In Situ Liquid Transmission Electron Microscopy. J. Phys. Chem. Lett. 2015, 6, 5051–5061.
- 22.Hodnik, N.; Dehm, G.; Mayrhofer, K.J.J. Importance and Challenges of Electrochemical in Situ Liquid Cell Electron Microscopy for Energy Conversion Research. Acc. Chem. Res. 2016, 49, 2015–2022.
- 23.Ross, F.M. Opportunities and Challenges in Liquid Cell Electron Microscopy. Science 2015, 350, aaa9886.
- 24.Peng, X.; Shangguan, J.; Zhang, Q.; Hauwiller, M.; Yu, H.; Nie, Y.; Bustillo, K.C.; Alivisatos, A.P.; Asta, M.; Zheng, H. Unveiling Corrosion Pathways of Sn Nanocrystals through High-Resolution Liquid Cell Electron Microscopy. Nano Lett. 2024, 24, 1168–1175.
- 25.Zheng, H.; Smith, R.K.; Jun, Y.W.; Kisielowski, C.; Dahmen, U.; Alivisatos, A.P. Observation of Single Colloidal Platinum Nanocrystal Growth Trajectories. Science 2009, 324, 1309–1312.
- 26.Zhang, Q.; Peng, X.; Nie, Y.; Zheng, Q.; Shangguan, J.; Zhu, C.; Bustillo, K.C.; Ercius, P.; Wang, L.; Limmer, D.T.; et al. Defect-Mediated Ripening of Core-Shell Nanostructures. Nat. Commun. 2022, 13, 2211.
- 27.Zheng, L.; Zhang, X.; Bustillo, K.C.; Yao, Y.; Zhao, L.; Zhu, M.; Li, W.; Zheng, H. Growth Mechanism of Core–Shell PtNi–Ni Nanoparticles Using in Situ Transmission Electron Microscopy. Nanoscale 2018, 10, 11281–11286.
- 28.Wu, J.; Gao, W.; Wen, J.; Miller, D.J.; Lu, P.; Zuo, J.-M.; Yang, H. Growth of Au on Pt Icosahedral Nanoparticles Revealed by Low-Dose In Situ TEM. Nano Lett. 2015, 15, 2711–2715.
- 29.Zheng, L.; Zhao, L.; Zhao, S.; Zhang, X.; Bustillo, K.C.; Yao, Y.; Yi, X.; Zhu, M.; Li, W.; Zheng, H. A Unique Pathway of PtNi Nanoparticle Formation Observed with Liquid Cell Transmission Electron Microscopy. Nanoscale 2020, 12, 1414–1418.
- 30.Li, D.; Nielsen, M.H.; Lee, J.R.I.; Frandsen, C.; Banfield, J.F.; De Yoreo, J.J. Direction-Specific Interactions Control Crystal Growth by Oriented Attachment. Science 2012, 336, 1014–1018.
- 31.Welch, D.A.; Woehl, T.J.; Park, C.; Faller, R.; Evans, J.E.; Browning, N.D. Understanding the Role of Solvation Forces on the Preferential Attachment of Nanoparticles in Liquid. ACS Nano 2015, 10, 181–187.
- 32.Wang, Y.; Peng, X.; Abelson, A.; Zhang, B.-K.; Qian, C.; Ercius, P.; Wang, L.-W.; Law, M.; Zheng, H. In Situ TEM Observation of Neck Formation During Oriented Attachment of PbSe Nanocrystals. Nano Res. 2019, 12, 2549–2553.
- 33.Zhu, C.; Liang, S.; Song, E.; Zhou, Y.; Wang, W.; Shan, F.; Shi, Y.; Hao, C.; Yin, K.; Zhang, T.; et al. In-Situ Liquid Cell Transmission Electron Microscopy Investigation on Oriented Attachment of Gold Nanoparticles. Nat. Commun. 2018, 9, 421.
- 34.Luo, B.; Smith, J.W.; Ou, Z.; Chen, Q. Quantifying the Self-Assembly Behavior of Anisotropic Nanoparticles Using Liquid-Phase Transmission Electron Microscopy. Acc. Chem. Res. 2017, 50, 1125–1133.
- 35.Kim, A.; Akkunuri, K.; Qian, C.; Yao, L.; Sun, K.; Chen, Z.; Vo, T.; Chen, Q. Direct Imaging of “Patch-Clasping” and Relaxation in Robust and Flexible Nanoparticle Assemblies. ACS Nano 2024, 18, 939–950.
- 36.Park, J.; Zheng, H.; Lee, W.C.; Geissler, P.L.; Rabani, E.; Alivisatos, A.P. Direct Observation of Nanoparticle Superlattice Formation by Using Liquid Cell Transmission Electron Microscopy. ACS Nano 2012, 6, 2078–2085.
- 37.Ou, Z.; Yao, L.; An, H.; Shen, B.; Chen, Q. Imaging How Thermal Capillary Waves and Anisotropic Interfacial Stiffness Shape Nanoparticle Supracrystals. Nat. Commun. 2020, 11, 4555.
- 38.Chen, Q.; Yuk, J.M.; Hauwiller, M.R.; Park, J.; Dae, K.S.; Kim, J.S.; Alivisatos, A.P. Nucleation, Growth, and Superlattice Formation of Nanocrystals Observed in Liquid Cell Transmission Electron Microscopy. MRS Bull. 2020, 45, 713–726.
- 39.Son, Y.; Kim, B.H.; Choi, B.K.; Luo, Z.; Kim, J.; Kim, G.-H.; Park, S.-J.; Hyeon, T.; Mehraeen, S.; Park, J. In Situ Liquid Phase TEM of Nanoparticle Formation and Diffusion in a Phase-Separated Medium. ACS Appl. Mater. Interfaces 2022, 14, 22810–22817.
- 40.Hong, J.; Bae, J.-H.; Jo, H.; Park, H.-Y.; Lee, S.; Hong, S.J.; Chun, H.; Cho, M.K.; Kim, J.; Kim, J.; et al. Metastable Hexagonal Close-Packed Palladium Hydride in Liquid Cell TEM. Nature 2022, 603, 631–636.
- 41.Crook, M.F.; Laube, C.; Moreno-Hernandez, I.A.; Kahnt, A.; Zahn, S.; Ondry, J.C.; Liu, A.; Alivisatos, A.P. Elucidating the Role of Halides and Iron during Radiolysis-Driven Oxidative Etching of Gold Nanocrystals Using Liquid Cell Transmission Electron Microscopy and Pulse Radiolysis. J. Am. Chem. Soc. 2021, 143, 11703–11713.
- 42.Hauwiller, M.R.; Ye, X.; Jones, M.R.; Chan, C.M.; Calvin, J.J.; Crook, M.F.; Zheng, H.; Alivisatos, A.P. Tracking the Effects of Ligands on Oxidative Etching of Gold Nanorods in Graphene Liquid Cell Electron Microscopy. ACS Nano 2020, 14, 10239–10250.
- 43.Woehl, T.J.; Park, C.; Evans, J.E.; Arslan, I.; Ristenpart, W.D.; Browning, N.D. Direct Observation of Aggregative Nanoparticle Growth: Kinetic Modeling of the Size Distribution and Growth Rate. Nano Lett. 2014, 14, 373–378.
- 44.Qin, F.; Wang, Z.; Wang, Z.L. Anomalous Growth and Coalescence Dynamics of Hybrid Perovskite Nanoparticles Observed by Liquid-Cell Transmission Electron Microscopy. ACS Nano 2016, 10, 9787–9793.
- 45.Talapin, D.V.; Rogach, A.L.; Haase, M.; Weller, H. Evolution of an Ensemble of Nanoparticles in a Colloidal Solution: Theoretical Study. J. Mater. Chem. B 2001, 105, 12278–12285.
- 46.Kang, S.; Kim, J.-H.; Lee, M.; Yu, J.W.; Kim, J.; Kang, D.; Baek, H.; Bae, Y.; Kim, B.H.; Kang, S.; et al. Real-Space Imaging of Nanoparticle Transport and Interaction Dynamics by Graphene Liquid Cell TEM. Sci. Adv. 2021, 7, eabi5419.
- 47.Kim, J.; Kang, D.; Kang, S.; Kim, B.H.; Park, J. Coalescence Dynamics of Platinum Group Metal Nanoparticles Revealed by Liquid-Phase Transmission Electron Microscopy. iScience 2022, 25, 104699.
- 48.Ma, X.; Lin, F.; Chen, X.; Jin, C. Unveiling Growth Pathways of Multiply Twinned Gold Nanoparticles by In Situ Liquid Cell Transmission Electron Microscopy. ACS Nano 2020, 14, 9594–9604.
- 49.Ma, X.; Lin, F.; Chen, X.; Jin, C. Synergy between Structure Characteristics and the Solution Chemistry in a Near/Non-Equilibrium Oxidative Etching of Penta-Twinned Palladium Nanorods. J. Phys. Chem. C 2021, 125, 4010–4020.
- 50.Zhang, Y.; Keller, D.; Rossell, M.D.; Erni, R. Formation of Au Nanoparticles in Liquid Cell Transmission Electron Microscopy: From a Systematic Study to Engineered Nanostructures. Chem. Mater. 2017, 29, 10518–10525.
- 51.Choi, B.K.; Kim, J.; Luo, Z.; Kim, J.; Kim, J.H.; Hyeon, T.; Mehraeen, S.; Park, S.; Park, J. Shape Transformation Mechanism of Gold Nanoplates. ACS Nano 2023, 17, 2007–2018.
- 52.Schneider, N.M.; Norton, M.M.; Mendel, B.J.; Grogan, J.M.; Ross, F.M.; Bau, H.H. Electron–Water Interactions and Implications for Liquid Cell Electron Microscopy. J. Phys. Chem. C 2014, 118, 22373–22382.
- 53.Woehl, T.J.; Moser, T.; Evans, J.E.; Ross, F.M. Electron-Beam-Driven Chemical Processes During Liquid Phase Transmission Electron Microscopy. MRS Bull. 2020, 45, 746–753.
- 54.Lifshitz, I.M.; Slyozov, V.V. The Kinetics of Precipitation from Supersaturated Solid Solutions. J. Phys. Chem. Solids 1961, 19, 35–50.
- 55.Viswanatha, R.; Sapra, S.; Satpati, B.; Satyam, P.V.; Dev, B.N.; Sarma, D.D. Understanding the Quantum Size Effects in ZnO Nanocrystals. J. Mater. Chem. 2004, 14, 661–668.
- 56.Sholl, D.S.; Skodje, R.T. Late-Stage Coarsening of Adlayers by Dynamic Cluster Coalescence. Phys. A 1996, 231, 631–647.
- 57.Meakin, P. Diffusion-Limited Droplet Coalescence. Phys. A 1990, 165, 1–18.
- 58.Viswanatha, R.; Sarma, D. D. Growth of Nanocrystals in Solution. In Nanomaterials Chemistry: Recent Developments and New Directions; Rao, C.N.R., Müller, A., Cheetham, A.K., Eds.; Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 139–170.
How to Cite
Ngo, T.; Yu, S.; Yang, H. Modelling the Growth and Aggregation of Gold Nanoparticles Using Liquid-Phase Transmission Electron Microscopy. Materials and Interfaces 2025, 2 (2), 201–212. https://doi.org/10.53941/mi.2025.100016.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


