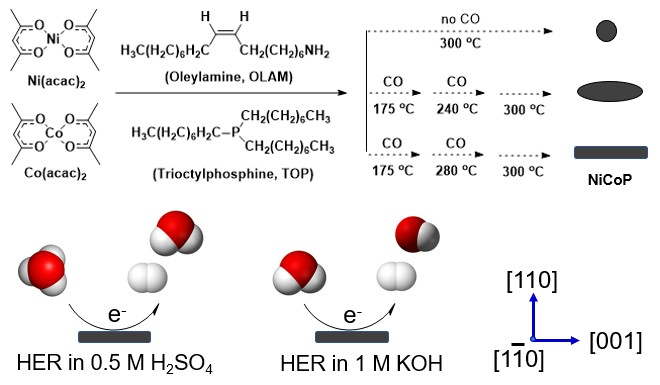
The development of efficient and cost-effective catalysts for hydrogen evolution reaction (HER) is crucial for the advancement of electrochemical water splitting technology. Here, we report a novel synthetic method for the preparation of single-crystalline NiCoP nanorods with tunable aspect ratios using a CO-assisted, trioctylphosphine (TOP)-mediated approach. The introduction of CO gas at different temperatures allows for the control of the nanorod growth, resulting in various aspect ratios while maintaining a hexagonal crystal structure and a composition of 1:1 Ni/Co as NiCoP. Our results demonstrate that the NiCoP nanorods with higher aspect ratios exhibit improved HER activity and stability, with the highest aspect ratio nanorods showing the lowest overpotential and Tafel slope in both acidic and alkaline media. This study highlights the importance of controlling the size and morphology of bimetallic phosphide nanoparticles to optimize their catalytic activity for HER, providing new insights into the design and optimization of nanostructured catalysts for electrochemical water splitting applications.
- Open Access
- Article
Carbon Monoxide-Assisted Synthesis of Nickel Cobalt Phosphide Nanorods for the Hydrogen Evolution Reaction
- Sarah York 1,
- Zachary R. Mansley 2, 3,
- Feng Wang 1,
- Yimei Zhu 3,
- Jingyi Chen 1, *
Author Information
Received: 23 Apr 2025 | Revised: 07 Jun 2025 | Accepted: 11 Jun 2025 | Published: 20 Jun 2025
Abstract
Graphical Abstract
References
- 1.Shi, Y.; Zhang, B. Recent Advances in Transition Metal Phosphide Nanomaterials: Synthesis and Applications in Hydrogen Evolution Reaction. Chem. Soc. Rev. 2016, 45, 1529–1541.
- 2.Pei, Y.; Cheng, Y.; Chen, J.; Smith, W.; Dong, P.; Ajayan, P.M.; Ye, M.; Shen, J. Recent Developments of Transition Metal Phosphides as Catalysts in the Energy Conversion Field. J. Mater. Chem. A 2018, 6, 23220–23243.
- 3.Wu, W.; Luo, S.; Huang, Y.; He, H.; Shen, P.K.; Zhu, J. Recent Advances in Transition Metal Phosphide-Based Heterostructure Electrocatalysts for the Oxygen Evolution Reaction. Mater. Chem. Front. 2024, 8, 1064–1083.
- 4.Li, Y.; Xin, T.; Cao, Z.; Zheng, W.; He, P.; Yoon Suk Lee, L. Optimized Transition Metal Phosphides for Direct Seawater Electrolysis: Current Trends. ChemSusChem 2024, 17, e202301926.
- 5.Ray, A.; Sultana, S.; Paramanik, L.; Parida, K.M. Recent Advances in Phase, Size, and Morphology-Oriented Nanostructured Nickel Phosphide for Overall Water Splitting. J. Mater. Chem. A 2020, 8, 19196–19245.
- 6.Lu, X.; Yan, K.; Yu, Z.; Wang, J.; Liu, R.; Zhang, R.; Qiao, Y.; Xiong, J. Transition Metal Phosphides: Synthesis Nanoarchitectonics, Catalytic Properties, and Biomass Conversion Applications. ChemSusChem 2024, 17, e202301687.
- 7.Sun, M.; Liu, H.; Qu, J.; Li, J. Earth-Rich Transition Metal Phosphide for Energy Conversion and Storage. Adv. Energy Mater. 2016, 6, 1600087.
- 8.Li, G.; Feng, Y.; Yang, Y.; Wu, X.; Song, X.; Tan, L. Recent Advances in Transition Metal Phosphide Materials: Synthesis and Applications in Supercapacitors. Nano Mater. Sci. 2024, 6, 174–192.
- 9.Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270.
- 10.Hansen, M.H.; Stern, L.-A.; Feng, L.; Rossmeisl, J.; Hu, X. Widely Available Active Sites on Ni2p for Electrochemical Hydrogen Evolution—Insights from First Principles Calculations. Phys. Chem. Chem. Phys. 2015, 17, 10823–10829.
- 11.Chung, Y.-H.; Gupta, K.; Jang, J.-H.; Park, H.S.; Jang, I.; Jang, J.H.; Lee, Y.-K.; Lee, S.-C.; Yoo, S.J. Rationalization of Electrocatalysis of Nickel Phosphide Nanowires for Efficient Hydrogen Production. Nano Energy 2016, 26, 496–503.
- 12.Xiao, J.; Lv, Q.; Zhang, Y.; Zhang, Z.; Wang, S. One-Step Synthesis of Nickel Phosphide Nanowire Array Supported on Nickel Foam with Enhanced Electrocatalytic Water Splitting Performance. RSC Adv. 2016, 6, 107859–107864.
- 13.Wang, Y.; Liu, L.; Zhang, X.; Yan, F.; Zhu, C.; Chen, Y. Self-Supported Tripod-Like Nickel Phosphide Nanowire Arrays for Hydrogen Evolution. J. Mater. Chem. A 2019, 7, 22412–22419.
- 14.Kibsgaard, J.; Tsai, C.; Chan, K.; Benck, J.D.; Nørskov, J.K.; Abild-Pedersen, F.; Jaramillo, T.F. Designing an Improved Transition Metal Phosphide Catalyst for Hydrogen Evolution Using Experimental and Theoretical Trends. Energy Environ. Sci. 2015, 8, 3022–3029.
- 15.Downes, C.A.; Van Allsburg, K.M.; Tacey, S.A.; Unocic, K.A.; Baddour, F.G.; Ruddy, D.A.; LiBretto, N.J.; O’Connor, M.M.; Farberow, C.A.; Schaidle, J.A.; et al. Controlled Synthesis of Transition Metal Phosphide Nanoparticles to Establish Composition-Dependent Trends in Electrocatalytic Activity. Chem. Mater. 2022, 34, 6255–6267.
- 16.Liu, J.; Wang, Z.; David, J.; Llorca, J.; Li, J.; Yu, X.; Shavel, A.; Arbiol, J.; Meyns, M.; Cabot, A. Colloidal Ni2-Xcoxp Nanocrystals for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2018, 6, 11453–11462.
- 17.Qian, C.; Kim, F.; Ma, L.; Tsui, F.; Yang, P.; Liu, J. Solution-Phase Synthesis of Single-Crystalline Iron Phosphide Nanorods/Nanowires. J. Am. Chem. Soc. 2004, 126, 1195–1198.
- 18.Park, J.; Koo, B.; Yoon, K.Y.; Hwang, Y.; Kang, M.; Park, J.-G.; Hyeon, T. Generalized Synthesis of Metal Phosphide Nanorods Via Thermal Decomposition of Continuously Delivered Metal-Phosphine Complexes Using a Syringe Pump. J. Am. Chem. Soc. 2005, 127, 8433–8440.
- 19.Brock, S.L.; Senevirathne, K. Recent Developments in Synthetic Approaches to Transition Metal Phosphide Nanoparticles for Magnetic and Catalytic Applications. J. Solid State Chem. 2008, 181, 1552–1559.
- 20.Zhang, Y.; Li, N.; Zhang, Z.; Li, S.; Cui, M.; Ma, L.; Zhou, H.; Su, D.; Zhang, S. Programmable Synthesis of Multimetallic Phosphide Nanorods Mediated by Core/Shell Structure Formation and Conversion. J. Am. Chem. Soc. 2020, 142, 8490–8497.
- 21.Thompson, D.; Hoffman, A.S.; Mansley, Z.R.; York, S.; Wang, F.; Zhu, Y.; Bare, S.R.; Chen, J. Synthesis of Amorphous and Various Phase-Pure Nanoparticles of Nickel Phosphide with Uniform Sizes via a Trioctylphosphine-Mediated Pathway. Inorg. Chem. 2024, 63, 18981–18991.
- 22.DeRight, R.E. The Decomposition of Formic Acid by Sulfuric Acid. J. Am. Chem. Soc. 1933, 55, 4761–4764.
- 23.Crystallography Open Database: Information Card for Entry 1008056. Available online: https://www.crystallogra phy.net/cod/1008056.html (access on 11 April 2025).
- 24.Sénateur, J.; Rouault, A.; L’Héritier, P.; Krumbügel-Nylund, M.A.; Fruchart, R.; Fruchart, D.; Convert, P.; Roudaut, E. La Selectivite Des Substitutions Dans Les Phases Mm’p Etude De L’ordre Par Diffraction Neutronique Dans Nicop. Mater. Res. Bull. 1973, 8, 229–238.
- 25.American Mineralogist Crystal Structure Database: Conip. Available online: https://rruff.geo.arizona.edu/AMS/res ult.php?key=_database_code_amcsd+0015998&viewing=html (access on 11 April 2025).
- 26.Chen, Y.; She, H.; Luo, X.; Yue, G.-H.; Peng, D.-L. Solution-Phase Synthesis of Nickel Phosphide Single-Crystalline Nanowires. J. Cryst. Growth 2009, 311, 1229–1233.
- 27.She, H.; Chen, Y.; Luo, X.; Yue, G.-H.; Peng, D.-L. Preparation of Anisotropic Transition Metal Phosphide Nanocrystals: The Case of Nickel Phosphide Nanoplatelets, Nanorods, and Nanowires. J. Nanosci. Nanotechnol. 2010, 10, 5175–5182.
- 28.Wu, J.; Gross, A.; Yang, H. Shape and Composition-Controlled Platinum Alloy Nanocrystals Using Carbon Monoxide as Reducing Agent. Nano Lett. 2011, 11, 798–802.
- 29.You, H.; Yang, S.; Ding, B.; Yang, H. Synthesis of Colloidal Metal and Metal Alloy Nanoparticles for Electrochemical Energy Applications. Chem. Soc. Rev. 2013, 42, 2880–2904.
- 30.Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291.
- 31.Lasia, A. Mechanism and Kinetics ofthe Hydrogen Evolution Reaction. Int. J. Hydrog. Energy 2019, 44, 19484–19518.
- 32.Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design Principles for Hydrogen Evolution Reaction Catalyst Materials. Nano Energy 2016, 29, 29–36.
- 33.Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801.
- 34.Trasatti, S.; Petrii, O. Real Surface Area Measurements in Electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376.
- 35.Morales, D.M.; Risch, M. Seven Steps to Reliable Cyclic Voltammetry Measurements for the Determination of Double Layer Capacitance. J. Phys. Energy 2021, 3, 034013.
- 36.McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357.
- 37.Manso, R.H.; Hong, J.; Wang, W.; Acharya, P.; Hoffman, A.S.; Tong, X.; Wang, F.; Greenlee, L.F.; Zhu, Y.; Bare, S.R.; et al. Revealing Structural Evolution of Nickel Phosphide-Iron Oxide Core–Shell Nanocatalysts in Alkaline Medium for the Oxygen Evolution Reaction. Chem. Mater. 2024, 36, 6440–6453.
- 38.Zhang, Y.; Gao, L.; Hensen, E.J.M.; Hofmann, J.P. Evaluating the Stability of Co2P Electrocatalysts in the Hydrogen Evolution Reaction for Both Acidic and Alkaline Electrolytes. ACS Energy Lett. 2018, 3, 1360–1365.
- 39.Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108.
- 40.Becke, A.D. ANew Mixing of Hartree-Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377.
- 41.Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305.
- 42.Helmich-Paris, B.; de Souza, B.; Neese, F.; Izsák, R. An Improved Chain of Spheres for Exchange Algorithm. J. Chem. Phys. 2021, 155, 104109.
How to Cite
York, S.; Mansley, Z. R.; Wang, F.; Zhu, Y.; Chen, J. Carbon Monoxide-Assisted Synthesis of Nickel Cobalt Phosphide Nanorods for the Hydrogen Evolution Reaction. Materials and Interfaces 2025, 2 (2), 226–238. https://doi.org/10.53941/mi.2025.100018.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


