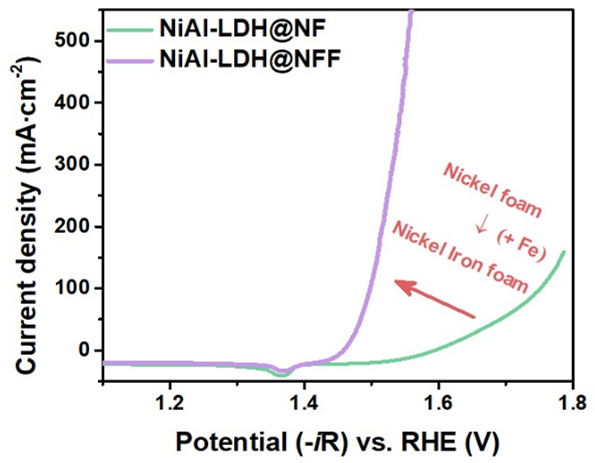
The development of sustainable energy that does not emit carbon pollutants is a major research topic toward minimizing waste generation and ecosystem degradation caused by the use of fossil fuels. Electrochemical water splitting is a semipermanent cycle that produces energy with zero carbon emissions. It is an innovative science and technology for a sustainable future for humanity and nature. However, its high operating potential due to the four-electron transfer process of the oxygen evolution reaction (OER) at the anode is the main factor hindering the overall reaction rate. Thus, given the importance of operating this cycle with high efficiency, studies have been extensively conducted to enhance the activity of transition-metal-based layered double hydroxide (LDH) catalysts. The use of metal foam as a substrate for LDH growth is considered the most effective method. However, most studies aimed at improving the performance of heterostructured catalysts have generally focused on controlling the active materials grown on the foam rather than the foam itself. Herein, we propose a new perspective on the role of foam, emphasizing that it is more than a mere supporting medium for growth. Density functional theory (DFT) calculations were performed to investigate the effects of NiFe foam (NFF) by modeling a heterostructure comprising NiAl-LDH and NFF. The calculation results demonstrated electron redistribution at the NiAl-LDH@NFF interface, which effectively influenced the OER performance and interfacial binding energy. Furthermore, we obtained insights into the role of foam by investigating changes in the OER overpotential caused by differences in the elements comprising the foam (Ni foam, 327 mV at 10 mA cm−2; NFF, 214 mV at 10 mA cm−2). This study affords flexibility in the utilization of metal foam-based heterostructured catalysts.
- Open Access
- Article
Growth of NiAl-LDH Nanoplates on NiFe Foam and Their Enhanced Electrochemical Properties for Oxygen Evolution Reaction
- Jaeyoung Lee 1, 2, †,
- Jae Ryeol Jeong 3, †,
- Yoojin Lee 4, †,
- Jinhoon Jang 1, †,
- Hongdeok Park 3,
- Yonghwan Jo 1,
- Jeong Woo Han 4, *,
- Min Hyung Lee 3, *,
- Taekyung Yu 1, *
Author Information
Received: 01 Sep 2025 | Revised: 19 Sep 2025 | Accepted: 29 Sep 2025 | Published: 30 Sep 2025
Abstract
Graphical Abstract
Keywords
NiFe foam | NiAl-LDH | oxygen evolution reaction | heterostructure modeling | density functional theory | substrate effect
References
- 1.Bhattacharya, M.; Paramati, S.R.; Ozturk, I.; Bhattacharya, S. The effect of renewable energy consumption on economic growth: Evidence from top 38 countries. Appl. Energy 2016, 162, 733–741.
- 2.Olatomiwa, L.; Mekhilef, S.; Ismail, M.S.; Moghavvemi, M. Energy management strategies in hybrid renewable energy systems: A review. Renew. Sustain. Energy Rev. 2016, 62, 821–835.
- 3.Chu, S., Cui, Y., & Liu, N. The path towards sustainable energy. Nature materials. 2017, 16(1), 16-22.
- 4.Vesborg, P.C.; Jaramillo, T.F. Addressing the terawatt challenge: Scalability in the supply of chemical elements for renewable energy. RSC Adv. 2012, 2, 7933–7947.
- 5.Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577.
- 6.Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074.
- 7.Jiao, Y.; Zheng, Y.; Davey, K.; Qiao, S.Z. Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom-doped graphene. Nat. Energy 2016, 1, 16130.
- 8.Reier, T.; Pawolek, Z.; Cherevko, S.; Bruns, M.; Jones, T.; Teschner, D.; Selve, S.; Bergmann, A.; Nong, H.N.; Schlögl, R.; et al. Molecular insight in structure and activity of highly efficient, low-Ir Ir–Ni oxide catalysts for electrochemical water splitting (OER). J. Am. Chem. Soc. 2015, 137, 13031–13040.
- 9.Forgie, R.; Bugosh, G.; Neyerlin, K.C.; Liu, Z.; Strasser, P. Bimetallic Ru electrocatalysts for the OER and electrolytic water splitting in acidic media. Electrochem. Solid-State Lett. 2010, 13, B36.
- 10.Li, B.Q.; Xia, Z.J.; Zhang, B.; Tang, C.; Wang, H.F.; Zhang, Q. Regulating p-block metals in perovskite nanodots for efficient electrocatalytic water oxidation. Nat. Commun. 2017, 8, 934.
- 11.Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013, 4, 2439.
- 12.Karmakar, A.; Karthick, K.; Sankar, S.S.; Kumaravel, S.; Madhu, R.; Kundu, S. A vast exploration of improvising synthetic strategies for enhancing the OER kinetics of LDH structures: A review. J. Mater. Chem. A 2021, 9, 1314–1352.
- 13.Liu, H.; Wang, Y.; Lu, X.; Hu, Y.; Zhu, G.; Chen, R.; Ma, L.; Zhu, H.; Tie, Z.; Liu, J.; et al. The effects of Al substitution and partial dissolution on ultrathin NiFeAl trinary layered double hydroxide nanosheets for oxygen evolution reaction in alkaline solution. Nano Energy 2017, 35, 350–357.
- 14.Yang, G.W.; Xu, C.L.; Li, H.L. Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem. Commun. 2008, 6537–6539.
- 15.Li, Y.; Yang, S.; Li, H.; Li, G.; Li, M.; Shen, L.; Yang, Z.; Zhou, A. Electrodeposited ternary iron-cobalt-nickel catalyst on nickel foam for efficient water electrolysis at high current density. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 694–702.
- 16.Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616.
- 17.Huang, L.A.; Shin, H.; Goddard III, W.A.; Wang, J. Photochemically deposited Ir-doped NiCo oxyhydroxide nanosheets provide highly efficient and stable electrocatalysts for the oxygen evolution reaction. Nano Energy 2020, 75, 104885.
- 18.Huang, H.; Jung, H.; Park, C.Y.; Kim, S.; Lee, A.; Jun, H.; Choi, J.; Han, J.W.; Lee, J. Surface conversion derived core-shell nanostructures of Co particles@ RuCo alloy for superior hydrogen evolution in alkali and seawater. Appl. Catal. B Environ. 2022, 315, 121554.
- 19.Park, B.J.; Wang, Y.; Lee, Y.; Noh, K.J.; Cho, A.; Jang, M.G.; Huang, R.; Lee, K.S.; Han, J.W. Effective screening route for highly active and selective Metal—Nitrogen-doped carbon catalysts in CO2 electrochemical reduction. Small 2021, 17, 2103705.
- 20.Liu, J.; Bak, J.; Roh, J.; Lee, K.S.; Cho, A.; Han, J.W.; Cho, E. Reconstructing the coordination environment of platinum single-atom active sites for boosting oxygen reduction reaction. Acs Catal. 2020, 11, 466–475.
- 21.Guo, C.; Jiao, Y.; Zheng, Y.; Luo, J.; Davey, K.; Qiao, S.Z. Intermediate modulation on noble metal hybridized to 2D metal-organic framework for accelerated water electrocatalysis. Chem 2019, 5, 2429–2441.
- 22.Hong, Y.R.; Dutta, S.; Jang, S.W.; Ngome Okello, O.F.; Im, H.; Choi, S.Y.; Han, J.W.; Lee, I.S. Crystal facet-manipulated 2D Pt nanodendrites to achieve an intimate heterointerface for hydrogen evolution reactions. J. Am. Chem. Soc. 2022, 144, 9033–9043.
- 23.Du, F.; Ling, X.; Wang, Z.; Guo, S.; Zhang, Y.; He, H.; Li, G.; Jiang, C.; Zhou, Y.; Zou, Z. Strained heterointerfaces in sandwich–like NiFe layered double hydroxides/Co1-xS for highly efficient and superior long–term durable oxygen evolution reaction. J. Catal. 2020, 389, 132–139.
- 24.Feng, L.; Du, Y.; Huang, J.; Cao, L.; Feng, L.; Feng, Y.; Liu, Q.; Yang, D.; Kajiyoshi, K. Nanoporous NiAl-LDH nanosheet arrays with optimized Ni active sites for efficient electrocatalytic alkaline water splitting. Sustain. Energy Fuels 2020, 4, 2850–2858.
- 25.McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987.
- 26.Anantharaj, S.; Kundu, S. Do the evaluation parameters reflect intrinsic activity of electrocatalysts in electrochemical water splitting? ACS Energy Lett. 2019, 4, 1260–1264.
- 27.Dang Van, C.; Kim, S.; Kim, M.; Lee, M.H. Effect of rare-earth element doping on NiFe-layered double hydroxides for water oxidation at ultrahigh current densities. ACS Sustain. Chem. Eng. 2023, 11, 1333–1343.
- 28.Grenier, P.; Houde, D.; Jandl, S.; Boatner, L.A. Soft-mode studies in KTa 0.93 Nb 0.07 O 3 with use of the time-resolved third-order optical susceptibility χ3. Phys. Rev. B 1993, 47, 1.
- 29.Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169.
- 30.Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953.
- 31.Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865.
- 32.Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188.
- 33.Pandya, N.Y.; Mevada, A.D.; Gajjar, P.N. Lattice dynamical and thermodynamic properties of FeNi3, FeNi and Fe3Ni invar materials. Comput. Mater. Sci. 2016, 123, 287–295.
- 34.Wang, C.; Wang, C.Y. Ni/Ni3Al interface: A density functional theory study. Appl. Surf. Sci. 2009, 255, 3669–3675.
- 35.Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist LR, K.J.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892.
- 36.Valdés, Á.; Qu, Z.W.; Kroes, G.J.; Rossmeisl, J.; Nørskov, J.K. Oxidation and photo-oxidation of water on TiO2 surface. J. Phys. Chem. C 2008, 112, 9872–9879.
- 37.Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360.
- 38.Liu, G.; Wang, B.; Wang, L.; Wei, W.; Quan, Y.; Wang, C.; Zhu, W.; Li, H.; Xia, J. MOFs derived FeNi3 nanoparticles decorated hollow N-doped carbon rod for high-performance oxygen evolution reaction. Green Energy Environ. 2022, 7, 423–431.
- 39.Hu, X.; Li, P.; Zhang, X.; Yu, B.; Lv, C.; Zeng, N.; Luo, J.; Zhang, Z.; Song, J.; Liu, Y. Ni-based catalyst derived from NiAl layered double hydroxide for vapor phase catalytic exchange between hydrogen and water. Nanomaterials 2019, 9, 1688.
- 40.Zhang, X.; Chen, X.; Jin, S.; Peng, Z.; Liang, C. Ni/Al2O3 catalysts derived from layered double hydroxide and their applications in hydrodeoxygenation of anisole. ChemistrySelect 2016, 1, 577–584.
- 41.Jo, W.K.; Moru, S.; Tonda, S. A green approach to the fabrication of a TiO 2/NiAl-LDH core–shell hybrid photocatalyst for efficient and selective solar-powered reduction of CO2 into value-added fuels. J. Mater. Chem. A 2020, 8, 8020–8032.
- 42.Koilraj, P.; Takemoto, M.; Tokudome, Y.; Bousquet, A.; Prevot, V.; Mousty, C. Electrochromic thin films based on NiAl layered double hydroxide nanoclusters for smart windows and low-power displays. ACS Appl. Nano Mater. 2020, 3, 6552–6562.
- 43.Yan, X.; Zhang, W.D.; Hu, Q.T.; Liu, J.; Li, T.; Liu, Y.; Gu, Z.G. Defects-rich nickel nanoparticles grown on nickel foam as integrated electrodes for electrocatalytic oxidation of urea. Int. J. Hydrogen Energy 2019, 44, 27664–27670.
- 44.Zhu, W.; Yue, X.; Zhang, W.; Yu, S.; Zhang, Y.; Wang, J.; Wang, J. Nickel sulfide microsphere film on Ni foam as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Commun. 2016, 52, 1486–1489.
- 45.Wang, Z.; Wang, F.; Tu, J.; Cao, D.; An, X.; Ye, Y. Nickel foam supported hierarchical mesoporous MnO2/Ni(OH)2 nanosheet networks for high performance supercapacitor electrode. Mater. Lett. 2016, 171, 10–13.
How to Cite
Lee, J.; Jeong, J. R.; Lee, Y.; Jang, J.; Park, H.; Jo, Y.; Han, J. W.; Lee, M. H.; Yu, T. Growth of NiAl-LDH Nanoplates on NiFe Foam and Their Enhanced Electrochemical Properties for Oxygen Evolution Reaction. Materials and Interfaces 2025, 2 (3), 363–374. https://doi.org/10.53941/mi.2025.100028.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


