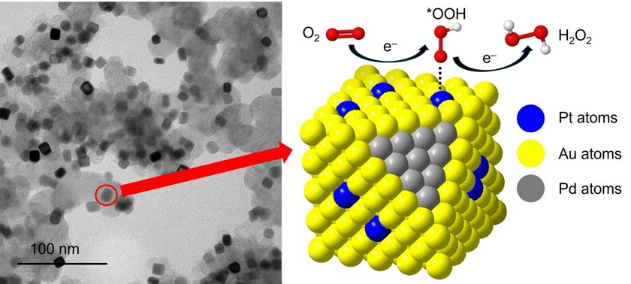
We report a versatile method based on seed-mediated growth for the facile synthesis of trimetallic Pd@PtxAu1−x core-shell nanocubes. By simply varying the feeding ratio between the Pt(II) and Au(III) precursors, the atomic ratio of Pt to Au in the shell and thereby the ensemble state of Pt atoms on the surface can be tuned to control the binding configuration of O2 molecules. Specifically, discrete Pt atoms on the surface promote the adsorption of O2 molecules in the Pauling configuration to enhance the catalytic selectivity of the nanoparticles toward H2O2 via the two-electron oxygen reduction reaction, with the Pd@Pt0.025Au0.975 nanocubes showing selectivity as high as 91% at 0.45 VRHE. This work offers a viable means to augment the electrocatalytic performance of alloy nanocrystals by controlling their surface compositions.
- Open Access
- Article
Trimetallic Pd@PtxAu1−x Core-Shell Nanocubes with Enhanced Selectivity toward H2O2 for the Oxygen Reduction Reaction
- Zhiqi Wang 1,
- Kei Kwan Li 1,
- Yong Ding 2,
- Younan Xia 1,3,*
Author Information
Received: 10 Oct 2025 | Revised: 30 Oct 2025 | Accepted: 07 Nov 2025 | Published: 13 Nov 2025
Abstract
Graphical Abstract
Keywords
alloy nanocrystals | core-shell nanocubes | H2O2 selectivity | oxygen reduction reaction | seed-mediated growth
References
- 1.
Luna, P.D.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 2019, 364, eaav3506.
- 2.
Lewis, R.J.; Hutchings, G.J. Recent advances in the direct synthesis of H2O2. ChemCatChem 2019, 11, 298–308.
- 3.
Siahrostami, S. H2O2 electrosynthesis and emerging applications challenges and opportunities: A computational perspective. Chem Catal. 2023, 3, 100568.
- 4.
Shin, H.; Lee, S.; Sung, Y.-E. Industrial-scale H2O2 electrosynthesis in practical electrochemical cell systems. Curr. Opin. Electrochem. 2023, 38, 101224.
- 5.
Wroblowa, H.S.; Pan, Y.-C.; Razumney, G. Electroreduction of oxygen: A new mechanistic criterion. J. Electroanal. Chem. 1976, 69, 195–201.
- 6.
Shen, R.; Chen, W.; Peng, Q.; Lu, S.; Zheng, L.; Cao, X.; Wang, Y.; Zhu, W.; Zhang, J.; Zhuang, Z.; et al. High-concentration single atomic Pt sites on hollow CuSx for selective O2 reduction to H2O2 in acid solution. Chem 2019, 5, 2099–2110.
- 7.
Zhang, J.; Ma, J.; Choksi, T.S.; Zhou, D.; Han, S.; Liao, Y.-F.; Yang, H.B.; Liu, D.; Zeng, Z.; Liu, W.; et al. Strong metal-support interaction boosts activity, selectivity and stability in electrosynthesis of H2O2. J. Am. Chem. Soc. 2022, 144, 2255–2263.
- 8.
Shen, H.; Janes, A.N.; Ross, R.D.; Kaiman, D.; Huang, J.; Song, B.; Schmidt, J.R.; Jin, S. Stable and selective electrosynthesis of hydrogen peroxide and the electro-Fenton process on CoSe2 polymorph catalysts. Energy Environ. Sci. 2020, 13, 4189–4203.
- 9.
Zhang, Y.; Lyu, Z.; Chen, Z.; Zhu, S.; Shi, Y.; Chen, R.; Xie, M.; Yao, Y.; Chi, M.; Shao, M.; et al. Maximizing the catalytic performance of Pd@AuxPd1−x nanocubes in H2O2 production by reducing shell thickness to increase compositional stability. Angew. Chem. Int. Ed. 2021, 60, 19643–19647.
- 10.
Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale structure design for high-performance Pt-based ORR catalysts. Adv. Mater. 2019, 31, 1802234.
- 11.
Zhang, G.; Wei, Q.; Yang, X.; Tavares, A.C.; Sun, S. RRDE experiments on noble-metal and noble-metal-free catalysts: Impact of loading on the activity and selectivity of oxygen reduction reaction in alkaline solution. Appl. Catal. B Environ. 2017, 206, 115–126.
- 12.
Gao, J.; Liu, B. Progress of electrochemical hydrogen peroxide synthesis over single atom catalysts. ACS Mater. Lett. 2020, 2, 1008–1024.
- 13.
Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143.
- 14.
Duan, Z.; Wang, G. Comparison of reaction energetics for oxygen reduction reactions on Pt(100), Pt(111), Pt/Ni(100) and Pt/Ni(111) surfaces: A first-principles study. J. Phys. Chem. C 2013, 117, 6284–6292.
- 15.
Choi, C.H.; Kwon, H.C.; Yook, S.; Shin, H.; Kim, H.; Choi, M. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt surface. J. Phys. Chem. C 2014, 118, 30063–30070.
- 16.
Jiang, K.; Zhao, J.; Wang, H. Catalyst design for electrochemical oxygen reduction toward hydrogen peroxide. Adv. Funct. Mater. 2020, 30, 2003321.
- 17.
Brimaud, S.; Engstfeld, A.K.; Alves, O.B.; Behm, R. Structure-reactivity correlation in the oxygen reduction reaction: Activity of structurally well-defined AuxPt1−x/Pt(111) monolayer surface alloys. J. Electroanal. Chem. 2014, 716, 71–79.
- 18.
Jin, M.; Liu, H.; Zhang, H.; Xie, Z.; Liu, J.; Xia, Y. Synthesis of Pd nanocrystals enclosed by {100} facets and with sizes <10 nm for application in CO oxidation. Nano Res. 2011, 4, 83–91.
- 19.
Peng, H.-C.; Xie, S.; Park, J.; Xia, X.; Xia, Y. Quantitative analysis of the coverage density of Br− ions on Pd{100} facets and its role in controlling the shape of Pd nanocrystals. J. Am. Chem. Soc. 2013, 135, 3780–3783.
- 20.
He, J.; Yu, H.; Xia, Y. Steady-state synthesis of colloidal metal nanocrystals. Mater. Interfaces 2025, 2, 213–225.
- 21.
Duchesne, P.N.; Li, Z.Y.; Deming, C.P.; Fung, V.; Zhao, X.; Yuan, J.; Regier, T.; Aldalbahi, A.; Almarhoon, Z.; Chen, S.; et al. Golden single-atom-site platinum electrocatalysts. Nat. Mater. 2018, 17, 1033–1039.
- 22.
Siahrostami, S.; Villegas, S.J.; Mostaghimi, A.H.B.; Back, S.; Farimani, A.B.; Wang, H.; Persson, K.A.; Montoya, J. A review on challenges and successes in atomic-scale design of catalysts for electrochemical synthesis of hydrogen peroxide. ACS Catal. 2020, 10, 7495–7511.
- 23.
Mei, D.; He, Z.D.; Zheng, Y.L.; Jiang, D.C.; Chen, Y.-X. Mechanistic and kinetic implications on the ORR on a Au(100) electrode: pH, temperature and H-D kinetic isotope effects. Phys. Chem. Chem. Phys. 2014, 16, 13762–13773.
- 24.
Ma, Z.; Cano, Z.P.; Yu, A.; Chen, Z.; Jiang, G.; Fu, X.; Yang, L.; Wu, T.; Bai, Z.; Lu, J. Enhancing oxygen reduction activity of Pt-based electrocatalysts: From theoretical mechanisms to practical methods. Angew. Chem. Int. Ed. 2020, 59, 18334–18348.
- 25.
Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 2018, 118, 2302–2312.
- 26.
Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Josson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892.
- 27.
Li, J.; Yin, H.-M.; Li, X.-B.; Okunishi, E.; Shen, Y.-L.; He, J.; Tang, Z.-K.; Wang, W.-X.; Yücelen, E.; Li, C.; et al. Surface evolution of a Pd-Pt-Au electrocatalyst for stable oxygen reduction. Nat. Energy 2017, 2, 17111–17116.
- 28.
Yu, S.; Yang, H. One the surface composition of molybdenum carbide nanoparticles for electrocatalytic applications. Mater. Interfaces 2024, 1, 3–12.
- 29.
Sun, W.; Min, B.; Wang, M.; Han, X.; Gao, Q.; Hwang, S.; Zhou, H.; Zhu, H. Catholic corrosion-induced structural evolution of CuNi electrocatalysts for enhanced CO2 reduction. Mater. Interfaces 2024, 1, 79–88.
- 30.
Zhou, J.; Shao, S.; Wei, Z.; Xia, X. Silver-platinum hollow nanoparticles for colorimetric lateral flow assay. Mater. Interfaces 2024, 1, 58–67.

This work is licensed under a Creative Commons Attribution 4.0 International License.


