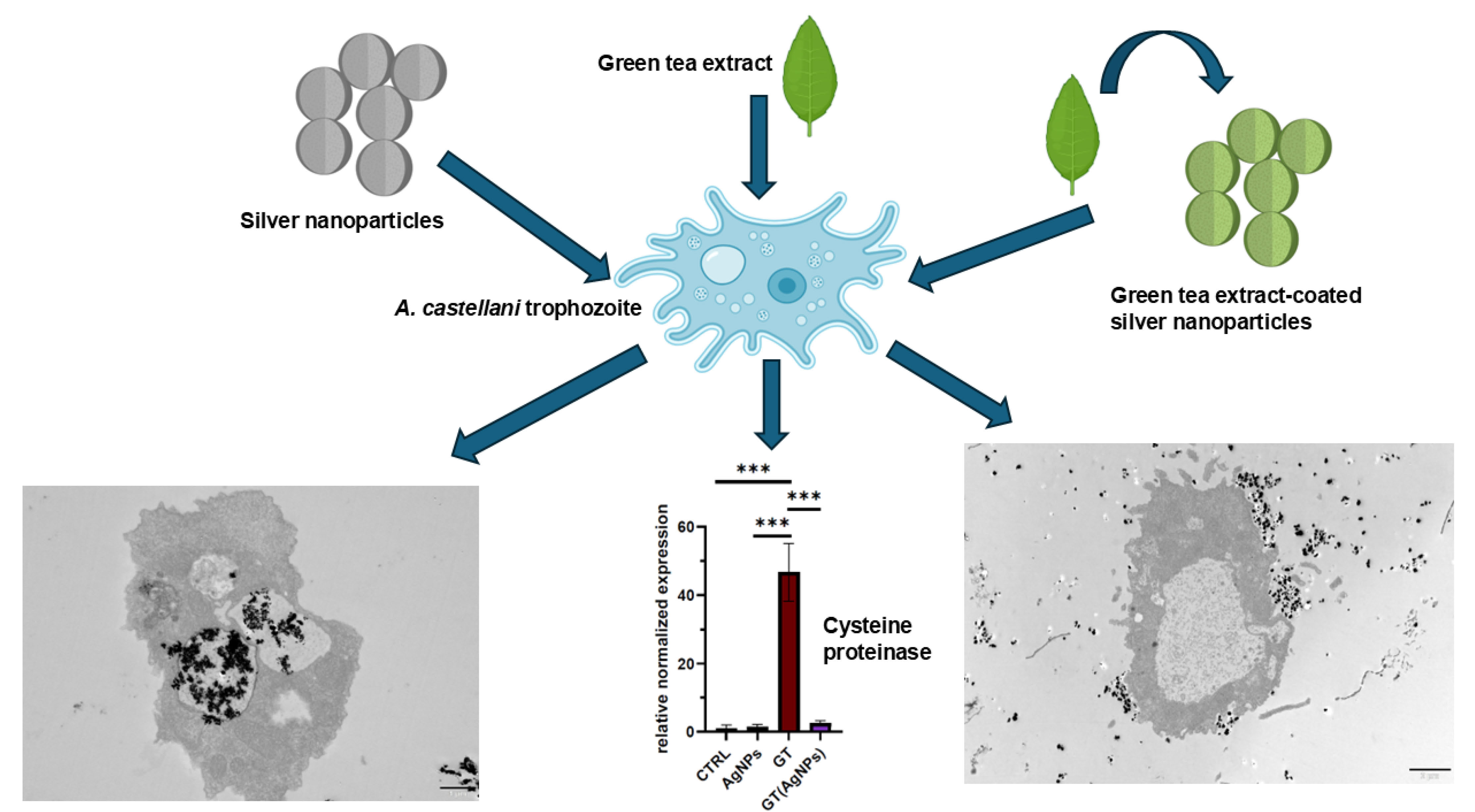
Efforts to utilise naturally derived substances in parasitology are gaining increasing interest. Among these are nanoparticles, whose antiparasitic properties may be enhanced when combined with plant extracts rich in bioactive compounds, beneficial to the host but toxic to parasites. This study evaluated whether silver nanoparticles coated with green tea (GT(AgNPs)), enhance the antiparasitic effects of silver nanoparticles (AgNPs) or green tea extract (GT) alone. Transmission electron microscopy revealed phagocytosis of nanoparticles, which accumulate in the vacuoles of amoebae. Notably, amoebae exposed to GT(AgNPs) exhibit cytoplasmic vacuolization and advanced cytoplasmic organelle disintegration, indicating strong toxicity even at lower levels of nanoparticle accumulation. Green tea extract alone is also toxic to amoebae, but without induction of cytosolic vacuolization. GT extract alone induced high expression of cysteine proteinase, paralleled by active autophagy. GT(AgNPs) did not have such effect on cysteine proteinase gene expression in amoebae. The mechanisms underlying the toxicity of silver nanoparticles and green tea appear to differ and are probably influenced by the condition of vacuole formation in trophozoites of Acanthamoeba castellanii.
- Open Access
- Article
Green Tea Anti-Acanthamoeba Activity Regulated by Silver Nanoparticles
- Ludmiła Szewczak 1,
- Mateusz Grotek 2,
- Julita Nowakowska 1,
- Mateusz Wdowiak 2,
- Jan Paczesny 2, *,
- Maria Doligalska 1, *
Author Information
Received: 13 May 2025 | Revised: 24 Jun 2025 | Accepted: 08 Aug 2025 | Published: 15 Aug 2025
Abstract
Graphical Abstract
Keywords
Acanthamoeba castellanii | silver nanoparticles | green tea | antiparasitic activity | amoeba cysteine proteinase | gene expression
References
- 1.Fan, S.; Shen, Y.; Qian, L. Social life of free-living amoebae in aquatic environment—comprehensive insights into interactions of free-living amoebae with neighbouring microorganisms. Front. Microbiol. 2024, 15, 1382075. https://doi.org/10.3389/fmicb.2024.1382075.
- 2.Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as Agents of Disease in Humans. Clin. Microbiol. Rev. 2003, 16, 273–307. https://doi.org/10.1128/CMR.16.2.273-307.2003.
- 3.Leońska-Duniec, A.; Adamska, M.; Skotarczyk, B. Molecular Identification of Free-living Amoebae Isolated from Artificial Water Bodies Located in Poland. Acta Protozool. 2015, 54, 77–84. https://doi.org/10.4467/16890027AP.15.006.2193.
- 4.Walvekar, S.; Anwar, A.; Anwar, A.; et al. Anti-amoebic potential of azole scaffolds and nanoparticles against pathogenic Acanthamoeba. Acta Trop. 2020, 211, 105618. https://doi.org/10.1016/j.actatropica.2020.105618.
- 5.Anwar, A.; Khan, N.A.; Siddiqui, R. Combating Acanthamoeba spp. cysts: What are the options? Parasit. Vectors 2018, 11, 26. https://doi.org/10.1186/s13071-017-2572-z.
- 6.Dhaka, A.; Suresh Chand Mali, S.C.; Sharma, S.; et al. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023, 6, 101108. https://doi.org/10.1016/j.rechem.2023.101108.
- 7.Abou Elez, R.M.M.; Attia, A.S.A.; Tolba, H.M.N.; et al. Molecular identification and antiprotozoal activity of silver nanoparticles on viability of Cryptosporidium parvum isolated from pigeons, pigeon fanciers and water. Sci. Rep. 2023, 13, 3109. https://doi.org/10.1038/s41598-023-30270-2.
- 8.Machado, L.F.; Sanfelice, R.A.; Bosqui, L.R.; et al. Biogenic silver nanoparticles reduce adherence, infection, and proliferation of toxoplasma gondii RH strain in HeLa cells without inflammatory mediators induction. Exp. Parasitol. 2020, 211, 107853. https://doi.org/10.1016/j.exppara.2020.107853.
- 9.Rai, M.; Ingle, A.P.; Paralikar, P.; et al. Recent advances in use of silver nanoparticles as antimalarial agents. Int. J. Pharm. 2017, 526, 254–270. https://doi.org/10.1016/j.ijpharm.2017.04.042.
- 10.Heidari-Kharaji, M.; Taheri, T.; Doroud, D.; et al. Enhanced paromomycin efficacy by solid lipid nanoparticle formulation against Leishmania in mice model. Parasite Immunol. 2016, 38, 599–608. https://doi.org/10.1111/pim.12340.
- 11.Pimentel-Acosta, C.A.; Morales-Serna, F.N.; Chávez-Sánchez, M.C.; et al. Efficacy of silver nanoparticles against the adults and eggs of monogenean parasites of fish. Parasitol. Res. 2019, 118, 1741–1749. https://doi.org/10.1007/s00436-019-06315-9.
- 12.Hamad, S.M.; Shnawa, B.H.; Jalil, P.J.; et al. Assessment of the Therapeutic Efficacy of Silver Nanoparticles against Secondary Cystic Echinococcosis in BALB/c Mice. Surfaces 2022, 5, 91–112. https://doi.org/10.3390/surfaces5010004.
- 13.Bahaaeldine, M.A.; El Garhy, M.; Fahmy, S.R.; et al. In vitro anti-Toxocara vitulorum effect of silver nanoparticles. J. Parasit. Dis. 2022, 46, 409–420. https://doi.org/10.1007/s12639-021-01464-0.
- 14.Goel, V.; Kaur, P.; Singla, L.D.; et al. Biomedical Evaluation of Lansium parasiticum Extract-Protected Silver Nanoparticles Against Haemonchus contortus, a Parasitic Worm. Front. Mol. Biosci. 2020, 7, 595646. https://doi.org/10.3389/fmolb.2020.595646.
- 15.Kaiaty, A.M.; Salib, F.A.; El-Gameel, S.M.; et al. Emerging alternatives to traditional anthelmintics: The in vitro antiparasitic activity of silver and selenium nanoparticles, and pomegranate (Punica granatum) peel extract against Haemonchus contortus. Trop. Anim. Health Prod. 2023, 55, 317. https://doi.org/10.1007/s11250-023-03722-0.
- 16.Zhang, P.; Gong, J.; Jiang, Y.; et al. Application of Silver Nanoparticles in Parasite Treatment. Pharmaceutics 2023, 15, 1783. https://doi.org/10.3390/pharmaceutics15071783.
- 17.Eker, F.; Duman, H.; Akdaşçi, E.; et al. Silver Nanoparticles in Therapeutics and Beyond: A Review of Mechanism Insights and Applications. Nanomaterials 2024, 14, 1618. https://doi.org/10.3390/nano14201618.
- 18.Hajizadeh, M.; Sarayan, M.S.; Taleghani, A.; et al. Evaluation of antimicrobial and antioxidant effects of silver nanoparticles synthesized with leaves of Lepidium draba L. JRRAS 2024, 17, 101004. https://doi.org/10.1016/j.jrras.2024.101004.
- 19.Rai, M.; Ingle, A.P.; Trzcińska-Wencel, J.; et al. Biogenic Silver Nanoparticles: What We Know and What Do We Need to Know? Nanomaterials 2021, 11, 2901. https://doi.org/10.3390/nano11112901.
- 20.Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; et al. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol Lett. 2017, 276, 11–20. https://doi.org/10.1016/j.toxlet.2017.05.007.
- 21.Xu, F.; Yao, Y.; Li, Y.; et al. A Review on the Application of Traditional to Modern Approaches of Chinese Herbal Veterinary Medicines: Current Status and Challenges. J. Food Biochem. 2024, 2024, 12. https://doi.org/10.1155/jfbc/5801408.
- 22.Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; et al. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. https://doi.org/10.3390/nu11020474.
- 23.Fakae, L.B.; Harun, M.S.R.; Ting, D.S.J.; et al. Camellia sinensis solvent extract, epigallocatechin gallate and caffeine confer trophocidal and cysticidal effects against Acanthamoeba castellanii. Acta Trop. 2023, 237, 106729. https://doi.org/10.1016/j.actatropica.2022.106729.
- 24.Fakae, L.B.; Stevenson, C.W.; Zhu, X.Q.; et al. In vitro activity of Camellia sinensis (green tea) against trophozoites and cysts of Acanthamoeba castellanii. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 59–72. https://doi.org/10.1016/j.ijpddr.2020.05.001.
- 25.Afonso, I.S.; Cardoso, B.; Nobrega, G.; et al. Green synthesis of nanoparticles from olive oil waste for environment. J. Environ. Chem. Eng. 2024, 12, 114022. https://doi.org/10.1016/j.jece.2024.114022.
- 26.Fahim, M.; Shahzaib, A.; Nishat, N.; et al. Green synthesis of silver nanoparticles: A comprehensive review of methods, influencing factors, and applications. JCIS Open 2024, 16, 100125. https://doi.org/10.1016/j.jciso.2024.100125.
- 27.Ahmed, S.; Ahmad, M.; Swami, B.L.; et al. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. https://doi.org/10.1016/j.jare.2015.02.007.
- 28.Wdowiak, M.; Raza, S.; Grotek, M.; et al Phage/nanoparticle cocktails for a biocompatible and environmentally friendly antibacterial therapy. Appl. Microbiol. Biotechnol. 2025, 109, 129, https://doi.org/10.1007/s00253-025-13526-x.
- 29.Anwar, A.; Ting, E.L.S.; Anwar, A.; et al. Antiamoebic activity of plant-based natural products and their conjugated silver nanoparticles against Acanthamoeba castellanii (ATCC 50492). AMB Express 2020, 10, 24. https://doi.org/10.1186/s13568-020-0960-9.
- 30.González-Fernández, S.; Lozano-Iturbe, V.; Menéndez, M.F.; et al. A Promising Antifungal and Antiamoebic Effect of Silver Nanorings, a Novel Type of AgNP. Antibiotics 2022, 11, 1054. https://doi.org/10.3390/antibiotics11081054.
- 31.Grün, A.; Scheid, P.; Hauröder, B.; et al. Assessment of the effect of silver nanoparticles on the relevant soil protozoan genus Acanthamoeba. J. Plant Nutr. Soil. Sci. 2017, 180, 602–613. https://doi.org/10.1002/jpln.201700277.
- 32.Hendiger, E.B.; Padzik, M.; Sifaoui, I.; et al. Silver Nanoparticles as a Novel Potential Preventive Agent against Acanthamoeba Keratitis. Pathogens 2020, 9, 350. https://doi.org/10.3390/pathogens9050350.
- 33.Kim, M.J.; Moon, E.K.; Jo, H.J.; et al. Phagocytosis-associated genes in Acanthamoeba castellanii feeding on Escherichia coli. Parasites Hosts Dis. 2023, 61, 397–404. https://doi.org/10.3347/PHD.23088.
- 34.Hong, Y.; Kang, J.M.; Joo, S.Y.; et al. Molecular and Biochemical Properties of a Cysteine Protease of Acanthamoeba castellanii. Korean J. Parasitol. 2018, 56, 409–418. https://doi.org/10.3347/kjp.2018.56.5.409.
- 35.Wang, Z.; Wu, D.; Tachibana, H.; et al. Identification and biochemical characterisation of Acanthamoeba castellanii cysteine protease 3. Parasit. Vectors 2020, 13, 592. https://doi.org/10.1186/s13071-020-04474-8.
- 36.Nakhjavani, M.; Nikkhah, V.; Sarafraz, M.M.; et al. Green synthesis of silver nanoparticles using green tea leaves: Experimental study on the morphological, rheological and antibacterial behaviour. Heat. Mass. Transfer. 2017, 53, 3201–3209. https://doi.org/10.1007/s00231-017-2065-9.
- 37.Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5-100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. https://doi.org/10.1039/c3ra44507k.
- 38.Červa, L. Amoebic meningoencephalitis: Axenic culture of Naegleria. Science 1969, 163, 576.
- 39.Kasprzak, W.; Mazur, T. Untersuchungen über die Pathogenität freilebender Amöeben von Badeplätzen in der Nähre von Poznań Polen für Mäuse [Free-living amoebae isolated from waters frequented by people in the vicinity of Poznań, Poland. Experimental studies in mice on the pathogenicity of the isolates]. Z. Tropenmed Parasitol. 1972, 23, 391–398.
- 40.Derda, M.; Hadaś, E.; Cholewiński, M.; et al. Artemisia annua L. as a plant with potential use in the treatment of acanthamoebiasis. Parasitol. Res. 2016, 115, 1635–1639. https://doi.org/10.1007/s00436-016-4902-z.
- 41.Köhsler, M.; Leitsch, D.; Müller, N.; et al. Validation of reference genes for the normalization of RT-qPCR gene expression in Acanthamoeba spp. Sci. Rep. 2020, 10, 10362. https://doi.org/10.1038/s41598-020-67035-0.
- 42.Taravaud, A.; Loiseau, P.M.; Pomel, S. In vitro evaluation of antimicrobial agents on Acanthamoeba sp. and evidence of a natural resilience to amphotericin B. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 328–336. https://doi.org/10.1016/j.ijpddr.2017.09.002.
- 43.Badirzadeh, A.; Alipour, M.; Najm, M.; et al. Potential therapeutic effects of curcumin coated silver nanoparticle in the treatment of cutaneous leishmaniasis due to Leishmania major in-vitro and in a murine model. JDDST 2022, 74, 103576. https://doi.org/10.1016/j.jddst.2022.103576.
- 44.Bajwa, H.U.R.; Khan, M.K.; Abbas, Z.; et al. Nanoparticles: Synthesis and Their Role as Potential Drug Candidates for the Treatment of Parasitic Diseases. Life 2022, 12, 750. https://doi.org/10.3390/life12050750.
- 45.Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; et al. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. https://doi.org/10.2147/IJN.S52634.
- 46.Dickson, A.; Cooper, E.; Fakae, L.B.; et al. In Vitro Growth- and Encystation-Inhibitory Efficacies of Matcha Green Tea and Epigallocatechin Gallate Against Acanthameoba Castellanii. Pathogens 2020, 9, 763. https://doi.org/10.3390/pathogens9090763.
- 47.Farokhzad, O.C.; Langer, R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv. Drug Deliv. Rev. 2006, 58, 1456–1459. https://doi.org/10.1016/j.addr.2006.09.011.
- 48.Sapsford, K.E.; Algar, W.R.; Berti, L.; et al. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. https://doi.org/10.1021/cr300143v.
- 49.Bowers, B.; Korn, E.D. The fine structure of Acanthamoeba castellanii. I. the trophozoite. J. Cell Biol. 1968, 39, 95–111. https://doi.org/10.1083/jcb.39.1.95.
- 50.Padzik, M.; Hendiger, E.B.; Chomicz, L.; et al. Tannic acid-modified silver nanoparticles as a novel therapeutic agent against Acanthamoeba. Parasitol. Res. 2018, 117, 3519–3525. https://doi.org/10.1007/s00436-018-6049-6.
- 51.Hendiger, E.B.; Padzik, M.; Żochowska, A.; et al. Tannic acid-modified silver nanoparticles enhance the anti-Acanthamoeba activity of three multipurpose contact lens solutions without increasing their cytotoxicity. Parasit. Vectors 2020, 13, 624. https://doi.org/10.1186/s13071-020-04453-z.
- 52.Roy, A.; Bulut, O.; Some, S.; et al. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. https://doi.org/10.1039/c8ra08982e.
- 53.Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. https://doi.org/10.1016/j.ecoenv.2023.114636.
- 54.Patlolla, A.K.; Hackett, D.; Tchounwou, P.B. Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol. Cell Biochem. 2015, 399, 257–268. https://doi.org/10.1007/s11010-014-2252-7.
- 55.Bao, H.; Yu, X.; Xu, C.; et al. New toxicity mechanism of silver nanoparticles: Promoting apoptosis and inhibiting proliferation. PLoS ONE 2015, 10, e0122535. https://doi.org/10.1371/journal.pone.0122535.
- 56.Cheng, X.; Zhang, W.; Ji, Y.; et al. Revealing silver cytotoxicity using Au nanorods/Ag shell nanostructures: Disrupting cell membrane and causing apoptosis through oxidative damage. RSC Adv. 2013, 3, 2296–2305. https://doi.org/10.1039/C2RA23131J.
- 57.Danila, O.O.; Berghian, A.S.; Dionisie, V.; et al. The effects of silver nanoparticles on behaviour, apoptosis and nitro-oxidative stress in offspring Wistar rats. Nanomedicine 2017, 12, 1455–1473. https://doi.org/10.2217/nnm-2017-0029.
- 58.Akter, M.; Sikder, M.T.; Rahman, M.M.; et al. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2017, 9, 1–16. https://doi.org/10.1016/j.jare.2017.10.008.
- 59.Raza, S.; Wdowiak, M.; Grotek, M.; et al. Enhancing the antimicrobial activity of silver nanoparticles against ESKAPE bacteria and emerging fungal pathogens by using tea extracts. Nanoscale Adv. 2023, 5, 5786–5798. https://doi.org/10.1039/d3na00220a.
- 60.Kliescikova, J.; Kulda, J.; Nohynkova, E. Stress-induced pseudocyst formation-a newly identified mechanism of protection against organic solvents in acanthamoebae of the T4 genotype. Protist 2011, 162, 58–69. https://doi.org/10.1016/j.protis.2010.03.006.
- 61.Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. https://doi.org/10.3390/ijms20020449.
- 62.Rohde, M.M.; Snyder, C.M.; Sloop, J.; et al. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part. Fibre Toxicol. 2021, 18, 37. https://doi.org/10.1186/s12989-021-00430-1.
- 63.Skalska, J.; Dąbrowska-Bouta, B.; Frontczak-Baniewicz, M.; et al. A Low Dose of Nanoparticulate Silver Induces Mitochondrial Dysfunction and Autophagy in Adult Rat Brain. Neurotox. Res. 2020, 38, 650–664. https://doi.org/10.1007/s12640-020-00239-4.
- 64.Wirwis, A.; Sadowski, Z. Green Synthesis of Silver Nanoparticles: Optimizing Green Tea Leaf Extraction for Enhanced Physicochemical Properties. ACS Omega 2023, 8, 30532–30549. https://doi.org/10.1021/acsomega.3c03775.
- 65.Yokozawa, T.; Dong, E. Influence of green tea and its three major components upon low-density lipoprotein oxidation. Exp Toxicol Pathol. 1997, 49, 329–335. https://doi.org/10.1016/S0940-2993(97)80096-6.
- 66.Sun, J.; Dong, S.; Li, J.; et al. A comprehensive review on the effects of green tea and its components on the immune function. Food Sci. Hum. Well. 2022, 11, 1143–1155. https://doi.org/10.1016/j.fshw.2022.04.008.
- 67.Chen, C.H.; Huang, J.M.; Wang, Y.J.; et al. Recent in vitro advances in the ocular antimicrobial agents against Acanthamoeba. Int. J. Parasitol. Drugs Drug Resist. 2025, 27, 100586. https://doi.org/10.1016/j.ijpddr.2025.100586.
- 68.Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 2021, 288, 36–55. https://doi.org/10.1111/febs.15453.
- 69.Leitsch, D.; Köhsler, M.; Marchetti-Deschmann, M.; et al. Major role for cysteine proteases during the early phase of Acanthamoeba castellanii encystment. Eukaryot. Cell. 2010, 9, 611–618. https://doi.org/10.1128/EC.00300-09.
Issue
Volume 1, Issue 1How to Cite
Szewczak, L.; Grotek, M.; Nowakowska, J.; Wdowiak, M.; Paczesny, J.; Doligalska, M. Green Tea Anti-Acanthamoeba Activity Regulated by Silver Nanoparticles. Parasitological Science 2025, 1 (1), 2.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


