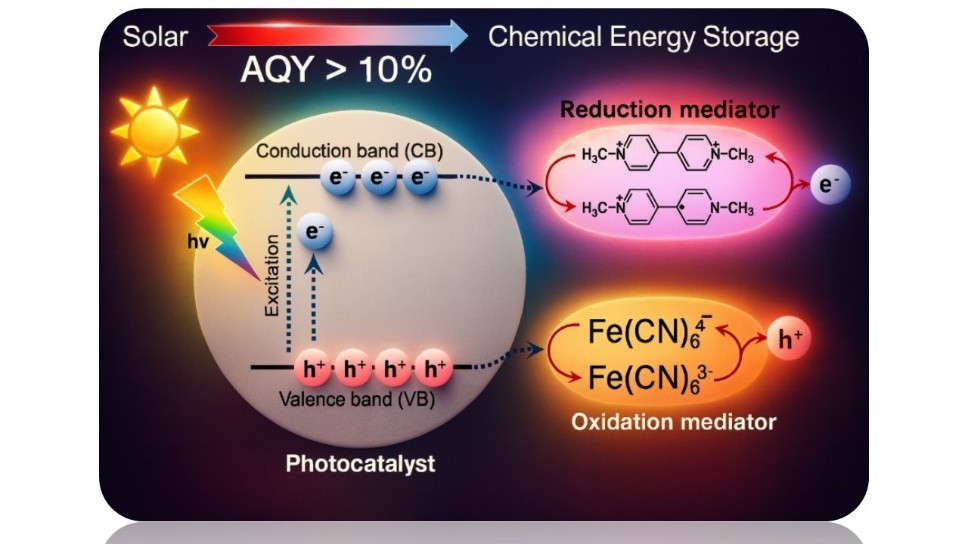
Despite the massive research effort in photocatalysis for solar fuels production, the efficiencies reached are still much lower than required for practical application. The present work proposes that to reach high apparent quantum yields in photocatalytic solar-to-chemicals conversions, mechanistically simple reactions should be used. Thus, instead of H2 generation or photocatalytic CO2 reduction, the present work reports data on the photocatalytic conversion of redox mediators that upon a single electron transfer convert the most stable form of a reversible redox pair into the highest energy species of the redox coupling. Specifically, by using two metal-organic frameworks as photocatalysts, the reduction of three bipyridinium dications and the oxidation of ferrocyanide and NADH was studied. Very high apparent quantum efficiencies were measured in most of the cases, the apparent quantum yields being particularly high for the conversion of methyl viologen to the corresponding radical cation and the photooxidation of ferrocyanide to ferricyanide. These findings outline a new strategy for solar energy conversion that emphasizes efficiency and mechanistic simplicity, shifting the focus from complex multi-step transformations such as water splitting or CO2 reduction to more straightforward reactions capable of storing highly efficiently sunlight in high-energy chemical states.
- Open Access
- Article
Photocatalytic Uphill Reactions with Apparent Quantum Efficiency over 10%
Author Information
Received: 21 Aug 2025 | Revised: 27 Oct 2025 | Accepted: 30 Oct 2025 | Published: 10 Nov 2025
Abstract
Graphical Abstract
Keywords
References
- 1.
Centi, G.; Perathoner, S. Towards solar fuels from water and CO2. ChemSusChem 2010, 3, 195–208.
- 2.Gust, D.; Moore, T.A.; Moore, A. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 2009, 42, 1890–1898.
- 3.Han, H.; Li, C. Photocatalysis in solar fuel production. Natl. Sci. Rev. 2015, 2, 145–147.
- 4.Chen, X.; Shen, S.; Guo, L.; et al. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570.
- 5.Kamat, P.V.; Bisquert, J. Solar fuels. Photocatalytic hydrogen generation. J. Phys. Chem. C 2013, 117, 14873–14875.
- 6.
Nishiyama, H.; Yamada, T.; Nakabayashi, M.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nat. Rev. Mater. 2021, 598, 304–307.
- 7.
Lu, Z.; Gao, J.; Rao, S.; et al. A multifunctional membrane based on TiO2/PCN-224 heterojunction with synergistic photocatalytic-photothermal activity under visible-light irradiation. Appl. Catal. B Environ. 2024, 342, 123374.
- 8.
Fang, S.; Rahaman, M.; Bharti, J.; et al. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers 2023, 3, 61.
- 9.
Khan, M.; Akmal, Z.; Tayyab, M.; et al. MOFs materials as photocatalysts for CO2 reduction: Progress, challenges and perspectives. Carbon Capture Sci. Technol. 2024, 11, 100191. https://doi.org/10.1016/j.ccst.2024.100191.
- 10.
Jiang, H.; Li, J.; Ren, W.; et al. Atomic-Level Engineering of Amide-bonded Ohmic-junctions for Synergistic Photocatalytic CO2-to-CO Conversion and H2O2 Production via Barrier-Free Charge Transfer in Pure H2O. Appl. Catal. B Environ. Energy 2025, 378, 125638.
- 11.Shen, H.; Yang, M.; Hao, L.; et al. Photocatalytic nitrogen reduction to ammonia: Insights into the role of defect engineering in photocatalysts. Nano Res. 2022, 15, 2773–2809.
- 12.Dhakshinamoorthy, A.; Li, Z.; Yang, S.; et al. Metal–organic framework heterojunctions for photocatalysis. Chem. Soc. Rev. 2024, 53, 3002–3035. https://doi.org/10.1039/D3CS00205E.
- 13.Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; et al. Metal–Organic Frameworks as Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Rev. 2023, 123, 445–490. https://doi.org/10.1021/acs.chemrev.2c00460.
- 14.
Li, K.; Peng, B.; Peng, T. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. Acs Catal. 2016, 6, 7485–7527.
- 15.Detz, R.; Reek, J.; Van Der Zwaan, B. The future of solar fuels: When could they become competitive? Energy Environ. Sci. 2018, 11, 1653–1669.
- 16.Kabir, E.; Kumar, P.; Kumar, S.; et al. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900.
- 17.Nayak, P.K.; Mahesh, S.; Snaith, H.J.; et al. Photovoltaic solar cell technologies: Analysing the state of the art. Nat. Rev. Mater. 2019, 4, 269–285.
- 18.Parida, B.; Iniyan, S.; Goic, R. A review of solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 15, 1625–1636.
- 19.Polman, A.; Knight, M.; Garnett, E.C.; et al. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, aad4424.
- 20.Yu, T.; He, W.; Zhang, Q.; et al. Advanced nanomaterials and characterization techniques for photovoltaic and photocatalysis applications. Acc. Mater. Res. 2023, 4, 507–521.
- 21.Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 1–17.
- 22.Bie, C.; Wang, L.; Yu, J. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574.
- 23.
Zhu, Z.; Liu, X.; Bao, C.; et al. How efficient could photocatalytic CO2 reduction with H2O into solar fuels be? Energy Convers. 2020, 222, 113236.
- 24.Schmidt, R. Photosensitized generation of singlet oxygen. Photochemistryphotobiology 2006, 82, 1161–1177.
- 25.Piguillem, S.V.; Gomez, G.E.; Tortella, G.R.; et al. Based Analytical Devices Based on Amino-MOFs (MIL-125, UiO-66, and MIL-101) as Platforms towards Fluorescence Biodetection Applications. Chemosensors 2024, 12, 208.
- 26.Mohamadpour, F.; Amani, A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024, 14, 20609–20645.
- 27.
Luu, C.L.; Nguyen, T.T.V.; Nguyen, T.; et al. Synthesis, characterization and adsorption ability of UiO-66-NH2. Adv. Nat.Sci. Nanosci. Nanotechnol. 2015, 6, 025004. https://doi.org/10.1088/2043-6262/6/2/025004.
- 28.
Kampouri, S.; Nguyen, T.N.; Spodaryk, M.; et al. Concurrent Photocatalytic Hydrogen Generation and Dye Degradation Using MIL-125-NH2 under Visible Light Irradiation. Adv. Funct. Mater. 2018, 28, 1806368. https://doi.org/10.1002/adfm.201806368.
- 29.Balčiūnas, S.; Pavlovaitė, D.; Kinka, M.; et al. Dielectric Spectroscopy of Water Dynamics in Functionalized UiO-66 Metal-Organic Frameworks. Molecules 2020, 25, 1962.
- 30.
Zhang, W.; Wang, L.; Zhang, J. Preparation of Ag/UiO-66-NH2 and its application in photocatalytic reduction of Cr(VI) under visible light. Res. Chem. Intermed. 2019, 45, 4801–4811. https://doi.org/10.1007/s11164-019-03865-6.
- 31.
Meng, J.; Chen, Q.; Lu, J.; et al. Z-Scheme Photocatalytic CO2 Reduction on a Heterostructure of Oxygen-Defective ZnO/Reduced Graphene Oxide/UiO-66-NH2 under Visible Light. ACS Appl. Mater. Interfaces 2019, 11, 550–562. https://doi.org/10.1021/acsami.8b14282.
- 32.
Wang, M.; Yang, L.; Yuan, J.; et al. Heterostructured Bi2S3@NH2-MIL-125(Ti) nanocomposite as a bifunctional photocatalyst for Cr(vi) reduction and rhodamine B degradation under visible light. RSC Adv. 2018, 8, 12459–12470. https://doi.org/10.1039/C8RA00882E.
- 33.
Kavun, V.; Uslamin, E.; van der Linden, B.; et al. Promoting Photocatalytic Activity of NH2-MIL-125(Ti) for H2 Evolution Reaction through Creation of TiIII- and CoI-Based Proton Reduction Sites. ACS Appl. Mater. Interfaces 2023, 15, 54590–54601. https://doi.org/10.1021/acsami.3c15490.
- 34.
Shi, X.; Lian, X.; Yang, D.; et al. Facet-engineering of NH2-UiO-66 with enhanced photocatalytic hydrogen production performance. Dalton Trans. 2021, 50, 17953–17959. https://doi.org/10.1039/D1DT03424C.
- 35.Sagara, T.; Tahara, H. Redox of viologen for powering and coloring. Chem. Rec. 2021, 21, 2375–2388.
- 36.Daeneke, T.; Uemura, Y.; Duffy, N.W.; et al. Aqueous dye-sensitized solar cell electrolytes based on the ferricyanide-ferrocyanide redox couple. Adv. Mater. 2012, 24, 1222–1225.
- 37.
Reyes, R.L.; Tanaka, K. The NAD+/NADH redox couple—Insights from the perspective of electrochemical energy transformation and biomimetic Chemistry. Kimika 2017, 28, 32–43.
- 38.Michaelis, L.; Hill, E.S. The viologen indicators. J. Gen. Physiol. 1933, 16, 859.
- 39.
Alvaro, M.; Carbonell, E.; Ferrer, B.; et al. Semiconductor behavior of a metal-organic framework (MOF). Chem. A Eur. J. 2007, 13, 5106–5112.
- 40.
De Miguel, M.; Ragon, F.; Devic, T.; et al. Evidence of Photoinduced Charge Separation in the Metal–Organic Framework MIL-125 (Ti)-NH2. ChemPhysChem 2012, 13, 3651–3654.
- 41.Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal–organic framework (MOF) compounds: Photocatalysts for redox reactions and solar fuel production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445.
- 42.de Lacey, A.L.; Fernández, V.M. pH-Dependent redox behaviour of asymmetric viologens. J. Electroanal. Chem. 1995, 399, 163–167.
- 43.
Collyer, S.D.; Davis, F.; Lucke, A.; et al. The electrochemistry of the ferri/ferrocyanide couple at a calix[4]resorcinarenetetrathiol-modified gold electrode as a study of novel electrode modifying coatings for use within electro-analytical sensors. J. Electroanal. Chem. 2003, 549, 119–127.
- 44.Qarah, N.A.; Basavaiah, K.; Abdulrahman, S.A. Spectrophotometric determination of ethionamide in pharmaceuticals using Folin–Ciocalteu reagent and iron(III)-ferricyanide as chromogenic agents. J. Taibah Univ. Sci. 2017, 11, 718–728.
- 45.
Saleh, F.S.; Rahman, M.R.; Okajima, T.; et al. Determination of formal potential of NADH/NAD+ redox couple and catalytic oxidation of NADH using poly (phenosafranin)-modified carbon electrodes. Bioelectrochemistry 2011, 80, 121–127.
- 46.Doménech, A.; García, H.; Doménech-Carbó, M.T.; et al. Electrochemistry of metal—organic frameworks: A description from the voltammetry of microparticles approach. J. Phys. Chem. C 2007, 111, 13701–13711.
- 47.Kosower, E.M.; Cotter, J.L. Stable free radicals. II. The reduction of 1-methyl-4-cyanopyridinium ion to methylviologen cation radical. J. Am. Chem. Soc. 1964, 86, 5524–5527.
- 48.Yamauchi, K.; Kawano, K.; Yatsuzuka, K.; et al. Viologen-Radical-Driven Hydrogen Evolution from Water Catalyzed by Co-NHC Catalysts: Radical Scavenging by Nitrate and Volmer-Heyrovsky-like CPET Pathway. J. Am. Chem. Soc. 2025, 147, 5602–5614.
- 49.Harriman, A.; Porter, G. Viologen/Platinum systems for hydrogen generation. J. Chem.Soc. Faraday Trans. 2 Mol. Phys. 1982, 78, 1937–1943.
- 50.
Li, Z.; Zhang, X.; Luo, Y.; et al. An electrochemical sensor based on the composite UiO-66-NH2/rGO for trace detection of Pb(II) and Cu(II). Chem. Phys. Lett. 2023, 830, 140825. https://doi.org/10.1016/j.cplett.2023.140825.
- 51.
Bravo Fuchineco, D.A.; Heredia, A.C.; Mendoza, S.M.; et al. Synthesis, Characterization and Catalytic Activity of UiO-66-NH2 in the Esterification of Levulinic Acid. Appl. Nano 2021, 2, 344–358.
- 52.Dan-Hardi, M.; Serre, C.; Frot, T.; et al. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. https://doi.org/10.1021/ja903726m.
- 53.
Wang, Z.; Inoue, Y.; Hisatomi, T.; et al. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat. Catal. 2018, 1, 756–763.
- 54.Wang, Q.; Nakabayashi, M.; Hisatomi, T.; et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting. Nature materials 2019, 18, 827–832.
- 55.
Zhao, Z.; Goncalves, R.V.; Barman, S.K.; et al. Electronic structure basis for enhanced overall water splitting photocatalysis with aluminum doped SrTiO3 in natural sunlight. Energy Environ. Sci. 2019, 12, 1385–1395.
- 56.
Kato, H.; Asakura, K.; Kudo, A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089.
- 57.Goto, Y.; Hisatomi, T.; Wang, Q.; et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2018, 2, 509–520.
- 58.Liu, T.; Pan, Z.; Kato, K.; et al. A general interfacial-energetics-tuning strategy for enhanced artificial photosynthesis. Nat. Commun. 2022, 13, 7783.
- 59.
Chen, D.; Chen, W.; Wu, Y.; et al. Covalent organic frameworks containing dual O2 reduction centers for overall photosynthetic hydrogen peroxide production. Angew. Chem. Int. Ed. 2023, 62, e202217479.

This work is licensed under a Creative Commons Attribution 4.0 International License.


