Oral regenerative medicine is crucial for restoring damaged enamel, dentin, pulp, and periodontium, yet conventional treatments fail to replicate native tissue hierarchy and bioactivity, causing poor integration and long-term failure. Nanomaterials, with unique properties (high surface-area-to-volume ratio, tunable chemistry, and stimuli responsiveness), address these issues. This review critically assesses the paradigm of nano-enabled strategies to achieve precise spatiotemporal control over the regenerative process. We focus on nanomaterials functional mechanisms within the oral milieu, including biomimetic mineralization, targeted bioactive cargo delivery to specific dental tissues, and intelligent modulation of cellular behavior and the local microenvironment. Inorganic nanoparticles (e.g., nano-hydroxyapatite, mesoporous silica) excel in biomimetic hardening and ion delivery; Organic nanocarriers (e.g., chitosan, PLGA, liposomes) offer superior biocompatibility and controlled release profiles; and biological nanoplatforms (e.g., exosomes, protein cages) provide unparalleled biorecognition and targeting. The review also evaluates translational hurdles (batch heterogeneity, rapid oral clearance, biosafety) and forecasts convergence with AI, 4D bioprinting, and gene editing to advance dentistry from repair to personalized restoration of oral tissues.
- Open Access
- Review
Nanomaterials in Oral Regeneration: Current Advances and Future Directions
Author Information
Received: 20 Aug 2025 | Revised: 20 Sep 2025 | Accepted: 11 Oct 2025 | Published: 24 Oct 2025
Abstract
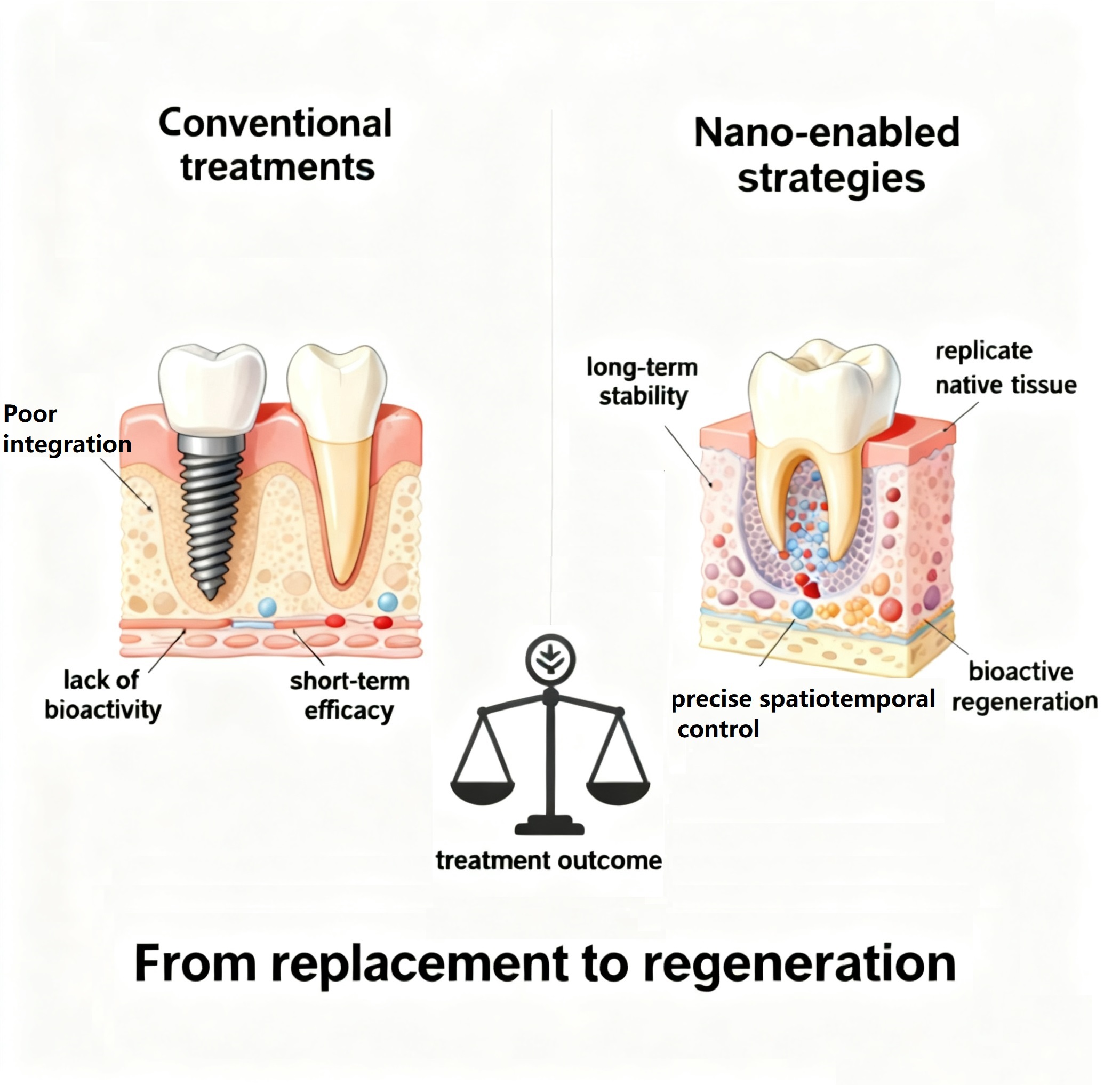
Graphical Abstract
Keywords
oral tissue regeneration | nanoparticles | AI | CRISPR-Cas9 | stimuli-responsive delivery
References
- 1.Ding, Q.; Cui, J.; Shen, H.; et al. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 13, e1669. https://doi.org/10.1002/wnan.1669.
- 2.Dang, G.; Wei, Y.; Wan, Q.; et al. Regulatory mechanisms and regeneration strategies of the soft–hard tissue interface in the human periodontium. BMEMat 2024, 2, e12069. https://doi.org/10.1002/bmm2.12069.
- 3.Olaru, M.; Sachelarie, L.; Calin, G. Hard Dental Tissues Regeneration-Approaches and Challenges. Materials 2021, 14, 2558. https://doi.org/10.3390/ma14102558.
- 4.Liu, H.; Li, B.; Gao, M.; et al. Restoration of human tooth enamel. BMEMat 2025, e12139. https://doi.org/10.1002/bmm2.12139.
- 5.Tang, W.; Fischer, N.G.; Kong, X.; et al. Hybrid coatings on dental and orthopedic titanium implants: Current advances and challenges. BMEMat 2024, 2, e12105. https://doi.org/10.1002/bmm2.12105.
- 6.Shakya, A.; Li, Y.; Chang, N.; et al. Supra-alveolar bone regeneration: Progress, challenges, and future perspectives. Compos. Part B Eng. 2024, 283, 111673. https://doi.org/10.1016/j.compositesb.2024.111673.
- 7.Xu, K.; Huang, R.; Li, X.; et al. Nanomaterial-based synergistic strategies for combating dental caries: Progress and perspectives. Nanoscale 2025, 17, 1874–1888. https://doi.org/10.1039/d4nr04515g.
- 8.Li, Y.; Cao, B.; Modali, S.; et al. Understanding the interactions between bone mineral crystals and their binding peptides derived from filamentous phage. Mater. Today Adv. 2022, 15, 100263. https://doi.org/10.1016/j.mtadv.2022.100263.
- 9.Kandhola, G.; Park, S.; Lim, J.-W.; et al. Nanomaterial-Based Scaffolds for Tissue Engineering Applications: A Review on Graphene, Carbon Nanotubes and Nanocellulose. Tissue Eng. Regen. Med. 2023, 20, 411–433. https://doi.org/10.1007/s13770-023-00530-3.
- 10.Hosseini, F.S.; Whitfield, T.; Orlando, J.D.; et al. Osteoinductive low-dose 3D porous calcium phosphate graphene oxide–integrated matrices enhance osteogenesis and mechanical properties. Proc. Natl. Acad. Sci. USA 2025, 122, e2427124122. https://doi.org/10.1073/pnas.2427124122.
- 11.Wang, Y.; Chang, L.; Gao, H.; et al. Nanomaterials-based advanced systems for photothermal / photodynamic therapy of oral cancer. Eur. J. Med. Chem. 2024, 272, 116508. https://doi.org/10.1016/j.ejmech.2024.116508.
- 12.Natsaridis, E.; Mouzoura, P.; Gkartziou, F.; et al. Development of growth factor-incorporating liposomes for integration into scaffolds as a method to improve tissue regeneration. Int. J. Dev. Biol. 2022, 66, 137–154. https://doi.org/10.1387/ijdb.210108sa.
- 13.Lin, S.; Xu, Z.; Liu, Y.; et al. Engineered Macrophage Membrane-Camouflaged Nanodecoys Reshape the Infectious Microenvironment for Efficient Periodontitis Treatment. ACS Nano 2025, 19, 15345–15362. https://doi.org/10.1021/acsnano.4c14305.
- 14.Pan, S.; Zhong, W.; Lan, Y.; et al. Pathology-Guided Cell Membrane-Coated Polydopamine Nanoparticles for Efficient Multisynergistic Treatment of Periodontitis. Adv. Funct. Mater. 2024, 34, 2312253. https://doi.org/10.1002/adfm.202312253.
- 15.Sedek, E.M.; Holiel, A.A. Next-Generation Strategies for Enamel Repair and Regeneration: Advances in Biomaterials and Translational Challenges. Tissue Eng. Regen. Med. 2025, 22, 771–789. https://doi.org/10.1007/s13770-025-00725-w.
- 16.Hou, X.; Zhang, L.; Zhou, Z.; et al. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. https://doi.org/10.3390/jfb13040187.
- 17.Fathy, S.M.; Abdelhafez, A.; Darwesh, F.A.; et al. Evaluation of incipient enamel-carious-like lesion treated with hydroxyapatite-chitosan nanocomposite hydrogel. J. World Fed. Orthod. 2024, 13, 211–220. https://doi.org/10.1016/j.ejwf.2024.04.001.
- 18.Talaat, S.; Hashem, A.A.; Abu-Seida, A.; et al. Regenerative potential of mesoporous silica nanoparticles scaffold on dental pulp and root maturation in immature dog’s teeth: A histologic and radiographic study. BMC Oral Health 2024, 24, 817. https://doi.org/10.1186/s12903-024-04368-6.
- 19.An, N.; Yan, X.; Qiu, Q.; et al. Human periodontal ligament stem cell sheets activated by graphene oxide quantum dots repair periodontal bone defects by promoting mitochondrial dynamics dependent osteogenic differentiation. J. Nanobiotechnol. 2024, 22, 133. https://doi.org/10.1186/s12951-024-02422-7.
- 20.Tan, Y.-J.; Li, X.; Zhang, W.-J.; et al. Mitochondria-targeted delivery of zinc-coordinated resveratrol nanoparticles rescues the osteogenic potential of periodontal ligament stem cells compromised by inflammation for periodontal wound healing. Chem. Eng. J. 2025, 503, 158296. https://doi.org/10.1016/j.cej.2024.158296.
- 21.Yan, M.; Xiao, B.; Yosick, A.; et al. Dose-dependent osteoimmunomodulatory effects of amorphous calcium phosphate nanoparticles promote 3D-printed scaffold-mediated bone regeneration. Bioact. Mater. 2025, 51, 197–210. https://doi.org/10.1016/j.bioactmat.2025.05.010.
- 22.Bordini, E.A.F.; Cassiano, F.B.; Bronze-Uhle, E.S.; et al. Chitosan in association with osteogenic factors as a cell-homing platform for dentin regeneration: Analysis in a pulp-in-a-chip model. Dent. Mater. 2022, 38, 655–669. https://doi.org/10.1016/j.dental.2022.02.004.
- 23.Tang, S.; Wang, W. Preparation and characterization of a novel composite membrane of natural silk fiber/nano-hydroxyapatite/chitosan for guided bone tissue regeneration. e-Polymers 2021, 21, 671–680. https://doi.org/10.1515/epoly-2021-0068.
- 24.Meng, L.; Shao, C.; Cui, C.; et al. Autonomous Self-Healing Silk Fibroin Injectable Hydrogels Formed via Surfactant-Free Hydrophobic Association. ACS Appl. Mater. Interfaces 2020, 12, 1628–1639. https://doi.org/10.1021/acsami.9b19415.
- 25.Pang, Y.; Kong, L.; Li, Y.; et al. PLGA/HA sustained-release system loaded with liraglutide for the treatment of diabetic periodontitis through inhibition of necroptosis. Mater. Today Bio 2025, 31, 101582. https://doi.org/10.1016/j.mtbio.2025.101582.
- 26.Fawzy, A.S.; Priyadarshini, B.M.; Selvan, S.T.; et al. Proanthocyanidins-Loaded Nanoparticles Enhance Dentin Degradation Resistance. J. Dent. Res. 2017, 96, 780–789. https://doi.org/10.1177/0022034517691757.
- 27.Capuano, N.; Amato, A.; Dell’Annunziata, F.; et al. Nanoparticles and Their Antibacterial Application in Endodontics. Antibiotics 2023, 12, 1690. https://doi.org/10.3390/antibiotics12121690.
- 28.An, S.; Gao, Y.; Chen, Y.; et al. CaSR as a Therapeutic Target and Tool in Human Dental Pulp: A Concise Review and Novel Hypothesis. Oral Health Prev. Dent. 2020, 18, 295–300. https://doi.org/10.3290/j.ohpd.a42688.
- 29.Azaryan, E.; Hanafi-Bojd, M.Y.; Alemzadeh, E.; et al. Effect of PCL/nHAEA nanocomposite to osteo/odontogenic differentiation of dental pulp stem cells. BMC Oral Health 2022, 22, 505. https://doi.org/10.1186/s12903-022-02527-1.
- 30.Gopinath, V.K.; Soumya, S.; Chakrapani, V.Y.; et al. Odontogenic differentiation of inflamed dental pulp stem cells (IDPSCs) on polycaprolactone (PCL) nanofiber blended with hydroxyapatite. Dent. Mater. J. 2021, 40, 312–321. https://doi.org/10.4012/dmj.2020-005.
- 31.Mehraliyeva, S.; Gasimov, E.; Rzayev, F.; et al. Development And Assessment of Quality Criteria of Routine Phytosomes in The Treatment of Periodontitis. Am. J. Biomed. Sci. Res. 2024, 22, 530–537. https://doi.org/10.34297/AJBSR.2024.22.002978.
- 32.Takeda, Y.S.; Xu, Q. Synthetic and nature-derived lipid nanoparticles for neural regeneration. Neural Regen. Res. 2015, 10, 689–690. https://doi.org/10.4103/1673-5374.156946.
- 33.Dantas, P.C.L.; Martins-Júnior, P.A.; Coutinho, D.C.O.; et al. Nanohybrid composed of graphene oxide functionalized with sodium hyaluronate accelerates bone healing in the tibia of rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111961. https://doi.org/10.1016/j.msec.2021.111961.
- 34.Absalan, F.; Seyed Sadjadi, M.; Farhadyar, N.; et al. Bone Tissue Engineering of HA/COL/GO Porous Nanocomposites with the Ability to Release Naproxen: Synthesis, Characterization, and In Vitro Study. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3260–3275. https://doi.org/10.1007/s10904-022-02283-3.
- 35.Xu, J.; Shi, H.; Luo, J.; et al. Advanced materials for enamel remineralization. Front. Bioeng. Biotechnol. 2022, 10, 985881. https://doi.org/10.3389/fbioe.2022.985881.
- 36.Lee, D.; Wufuer, M.; Kim, I.; et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021, 11, 746. https://doi.org/10.1038/s41598-020-80608-3.
- 37.Whitehouse, L.L.; Thomson, N.H.; Do, T.; et al. Bioactive molecules for regenerative pulp capping. Eur. Cell Mater. 2021, 42, 415–437. https://doi.org/10.22203/eCM.v042a26.
- 38.Liu, H.; Chen, B.; Liu, Y.; et al. Application of polysaccharide materials in the prevention and treatment of oral diseases. J. Drug Deliv. Sci. Technol. 2024, 93, 105331. https://doi.org/10.1016/j.jddst.2023.105331.
- 39.Wei, X.; Xu, H.; Zhou, M.; et al. Chemically modified microRNA delivery via DNA tetrahedral frameworks for dental pulp regeneration. J. Nanobiotechnol. 2024, 22, 150. https://doi.org/10.1186/s12951-024-02393-9.
- 40.Chopra, A.; Bhuvanagiri, G.; Natu, K.; et al. Role of CRISPR-Cas systems in periodontal disease pathogenesis and potential for periodontal therapy: A review. Mol. Oral Microbiol. 2025, 40, 1–16. https://doi.org/10.1111/omi.12483.
- 41.Man, K.; Eisenstein, N.M.; Hoey, D.A.; et al. Bioengineering extracellular vesicles: Smart nanomaterials for bone regeneration. J. Nanobiotechnol. 2023, 21, 137. https://doi.org/10.1186/s12951-023-01895-2.
- 42.Elkhouly, M.A.; Emara, M.M.; Nady, N.; et al. Bioinspired enamel repair via inorganic ionic polymerization using calcium phosphate ionic clusters and nano-hydroxyapatite. Sci. Rep. 2025, 15, 20207. https://doi.org/10.1038/s41598-025-06434-7.
- 43.Dai, D.; Wang, J.; Xie, H.; et al. An epigallocatechin gallate-amorphous calcium phosphate nanocomposite for caries prevention and demineralized enamel restoration. Mater. Today Bio 2023, 21, 100715. https://doi.org/10.1016/j.mtbio.2023.100715.
- 44.Balkaya, H.; Demirbuğa, S.; Dayan, S.; et al. Investigation of the physicochemical, cytotoxic, and antimicrobial properties of a resin-based pulp capping material incorporated with calcium fructoborate-loaded mesoporous silica nanoparticles. Dent. Mater. 2025, 41, 1080–1090. https://doi.org/10.1016/j.dental.2025.06.019.
- 45.Li, Q.; Wang, Z. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. https://doi.org/10.2147/ijn.S245608.
- 46.Ducret, M.; Costantini, A.; Gobert, S.; et al. Fibrin-based scaffolds for dental pulp regeneration: From biology to nanotherapeutics. Eur. Cell Mater. 2021, 41, 1–14. https://doi.org/10.22203/eCM.v041a01.
- 47.Lin, Y.; Li, Q.; Wang, L.; et al. Advances in regenerative medicine applications of tetrahedral framework nucleic acid-based nanomaterials: An expert consensus recommendation. Int. J. Oral Sci. 2022, 14, 51. https://doi.org/10.1038/s41368-022-00199-9.
- 48.Zheng, C.Y.; Chu, X.Y.; Gao, C.Y.; et al. TAT&RGD Peptide-Modified Naringin-Loaded Lipid Nanoparticles Promote the Osteogenic Differentiation of Human Dental Pulp Stem Cells. Int. J. Nanomed. 2022, 17, 3269–3286. https://doi.org/10.2147/ijn.S371715.
- 49.Cao, J.; Song, Z.; Du, T.; et al. Antimicrobial materials based on photothermal action and their application in wound treatment. Burn. Trauma 2024, 12, tkae046. https://doi.org/10.1093/burnst/tkae046.
- 50.Lin, H.P.; Tu, H.P.; Hsieh, Y.P.; et al. Controlled release of lovastatin from poly(lactic-co-glycolic acid) nanoparticles for direct pulp capping in rat teeth. Int. J. Nanomed. 2017, 12, 5473–5485. https://doi.org/10.2147/ijn.S138410.
- 51.Lee, Y.H.; Kim, J.S.; Kim, J.E.; et al. Nanoparticle mediated PPARγ gene delivery on dental implants improves osseointegration via mitochondrial biogenesis in diabetes mellitus rat model. Nanomedicine 2017, 13, 1821–1832. https://doi.org/10.1016/j.nano.2017.02.020.
- 52.Chen, Y.; Ma, Y.; Yang, X.; et al. The Application of Pulp Tissue Derived-Exosomes in Pulp Regeneration: A Novel Cell-Homing Approach. Int. J. Nanomed. 2022, 17, 465–476. https://doi.org/10.2147/ijn.S342685.
- 53.Wu, X.; Hu, Y.; Sheng, S.; et al. DNA-based hydrogels for bone regeneration: A promising tool for bone organoids. Mater. Today Bio 2025, 31, 101502. https://doi.org/10.1016/j.mtbio.2025.101502.
- 54.Nishida, E.; Miyaji, H.; Kato, A.; et al. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265–2277. https://doi.org/10.2147/ijn.S104778.
- 55.Kim, B.N.; Ko, Y.G.; Yeo, T.; et al. Guided Regeneration of Rabbit Calvarial Defects Using Silk Fibroin Nanofiber-Poly(glycolic acid) Hybrid Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 5266–5272. https://doi.org/10.1021/acsbiomaterials.9b00678.
- 56.Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. https://doi.org/10.1016/j.actbio.2019.03.021.
- 57.Rosa, V.; Silikas, N.; Yu, B.; et al. Guidance on the assessment of biocompatibility of biomaterials: Fundamentals and testing considerations. Dent. Mater. 2024, 40, 1773–1785. https://doi.org/10.1016/j.dental.2024.07.020.
- 58.Piotrowski-Daspit, A.S.; Bracaglia, L.G.; Eaton, D.A.; et al. Enhancing in vivo cell and tissue targeting by modulation of polymer nanoparticles and macrophage decoys. Nat. Commun. 2024, 15, 4247. https://doi.org/10.1038/s41467-024-48442-7.
- 59.Lin, S.; Cui, T.; Jiang, Y.; et al. Microenvironment-responsive NIR-IIb multifunctional nanozyme platform for bacterial imaging and specialized anti-anaerobic bacteria periodontal therapy. J. Nanobiotechnol. 2025, 23, 189. https://doi.org/10.1186/s12951-025-03270-9.
- 60.Teubl, B.J.; Stojkovic, B.; Docter, D.; et al. The effect of saliva on the fate of nanoparticles. Clin. Oral Investig. 2018, 22, 929–940. https://doi.org/10.1007/s00784-017-2172-5.
- 61.Lu, B.; Wang, J.; Hendriks, A.J.; et al. Clearance of nanoparticles from blood: Effects of hydrodynamic size and surface coatings. Environ.Sci. Nano 2024, 11, 406–417. https://doi.org/10.1039/D3EN00812F.
- 62.Pokrowiecki, R.; Wojnarowicz, J.; Zareba, T.; et al. Nanoparticles And Human Saliva: A Step Towards Drug Delivery Systems for Dental and Craniofacial Biomaterials. Int. J. Nanomed. 2019, 14, 9235–9257. https://doi.org/10.2147/ijn.S221608.
- 63.Liu, L.; Yao, W.; Rao, Y.; et al. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. https://doi.org/10.1080/10717544.2017.1279238.
- 64.Govindarajan, D.K.; Mohanarangam, M.; Kadirvelu, L.; et al. Biofilms and oral health: Nanotechnology for biofilm control. Discov. Nano 2025, 20, 114. https://doi.org/10.1186/s11671-025-04299-3.
- 65.Zhang, J.; Liu, W.; Shi, L.; et al. The Effects of Drug Addiction and Detoxification on the Human Oral Microbiota. Microbiol. Spectr. 2023, 11, e0396122. https://doi.org/10.1128/spectrum.03961-22.
- 66.Li, C.; Wang, Q. Advanced NIR-II Fluorescence Imaging Technology for In Vivo Precision Tumor Theranostics. Adv. Ther. 2019, 2, 1900053. https://doi.org/10.1002/adtp.201900053.
- 67.Yin, X.; Zhao, B.; Chen, L.; et al. Octahedral Fe3O4 nanozymes penetrate and remove biofilms on implants via photomagnetic response. Coatings 2025, 15, 728. https://doi.org/10.3390/coatings15060728.
- 68.Tomeh, M.A.; Zhao, X. Recent Advances in Microfluidics for the Preparation of Drug and Gene Delivery Systems. Mol. Pharm. 2020, 17, 4421–4434. https://doi.org/10.1021/acs.molpharmaceut.0c00913.
- 69.van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2022, 11, e2100639. https://doi.org/10.1002/adhm.202100639.
- 70.Aafreen, S.; Feng, J.; Wang, W.; et al. Theranostic extracellular vesicles: A concise review of current imaging technologies and labeling strategies. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 107–132. https://doi.org/10.20517/evcna.2023.01.
- 71.Nawaz, A.; Ariffin, N.S.; Wong, T.W. Functionalized chitosan as nano-delivery platform for CRISPR-Cas9 in cancer treatment. Asian J. Pharm. Sci. 2025, 20, 101041. https://doi.org/10.1016/j.ajps.2025.101041.
- 72.Bugueno, I.M.; Rey, T.; Jimenez-Armijo, A.; et al. Rare dentin defects: Understanding the pathophysiological mechanisms of COLXVA1 mutations. Genes Dis. 2024, 11, 101303. https://doi.org/10.1016/j.gendis.2024.101303.
- 73.Park, S.Y.; Kim, K.H.; Kim, S.; et al. BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry. Pharmaceutics 2019, 11, 393. https://doi.org/10.3390/pharmaceutics11080393.
- 74.Ruslan, D.S. AI-powered nanodevices for real-time monitoring of physiological parameters. Int. J. Sci. Res. 2022, 8, 22–50.
- 75.Chugh, V.; Basu, A.; Kaushik, A.; et al. Employing nano-enabled artificial intelligence (AI)-based smart technologies for prediction, screening, and detection of cancer. Nanoscale 2024, 16, 5458–5486. https://doi.org/10.1039/d3nr05648a.
- 76.Akkaş, T.; Reshadsedghi, M.; Şen, M.; et al. The Role of Artificial Intelligence in Advancing Biosensor Technology: Past, Present, and Future Perspectives. Adv. Mater. 2025, 37, e2504796. https://doi.org/10.1002/adma.202504796.
- 77.Qiu, Q.; Li, S.; Zhang, J.; et al. Lab-in-the-loop machine learning for brain-targeting delivery system design. Cell Biomater. 2025, 1, 100130. https://doi.org/10.1016/j.celbio.2025.100130.
How to Cite
Xu, S.; Liang, P.; Zhong, L.; Fu, X.; Li, Y.; Wang, Y. Nanomaterials in Oral Regeneration: Current Advances and Future Directions. Regenerative Medicine and Dentistry 2025, 2 (4), 16. https://doi.org/10.53941/rmd.2025.100016.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


