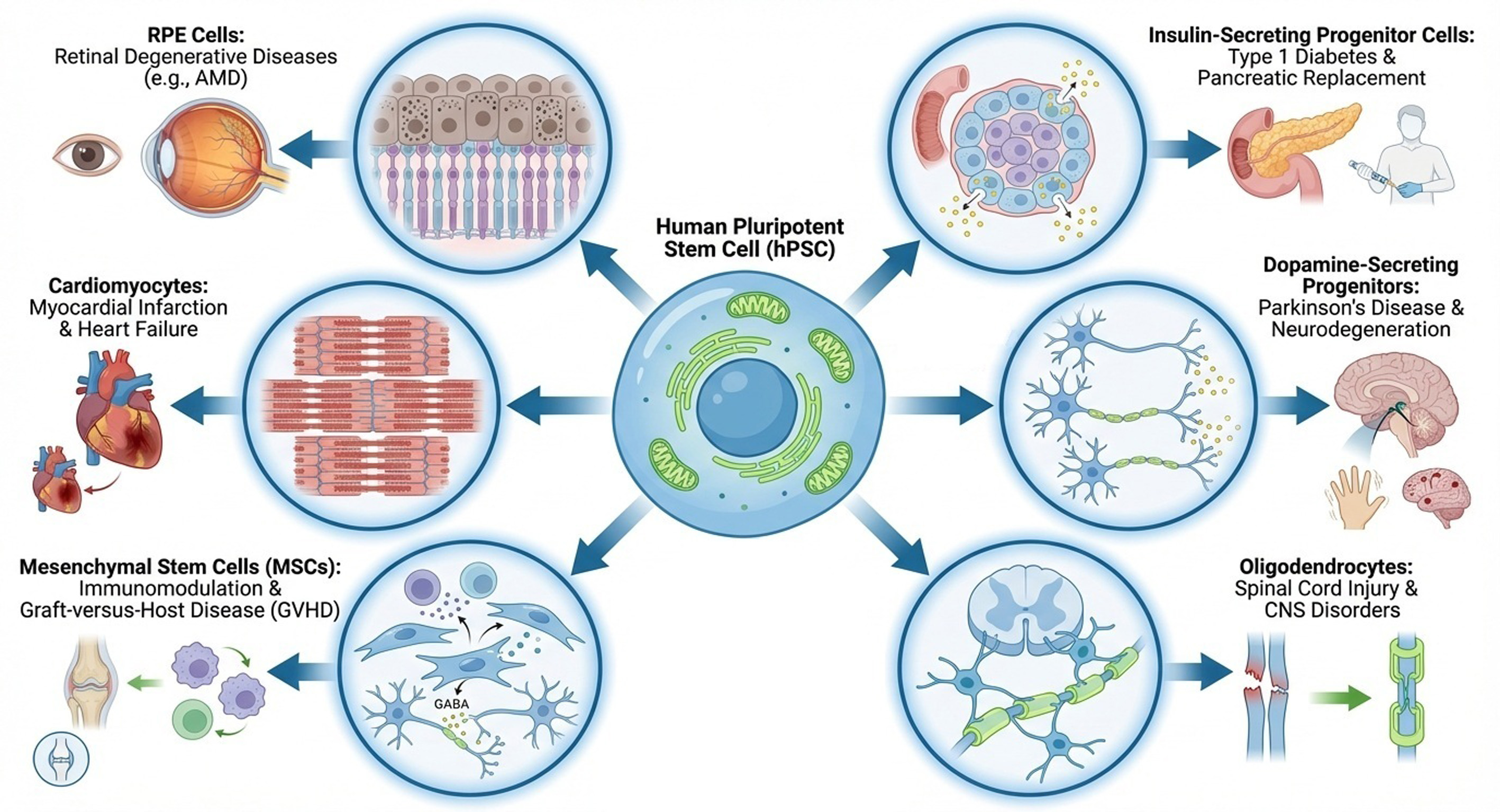
Human pluripotent stem cells (hPSCs) can be differentiated into any type of tissue cells in the human body. Several clinical trials have been started for the medical care of patients with organ failure, such as those with retinal degeneration diseases (including age-related macular degeneration), Parkinson’s disease, type 1 diabetes, spinal cord injury, epilepsy, myocardial infarction, graft-versus-host disease (GvHD), and cancer, using specific hPSC-derived tissue cells such as retinal pigment epithelium cells, insulin-secreting progenitor cells, dopamine-secreting progenitor cells, oligodendrocytes, GABAergic neurons, cardiomyocytes, mesenchymal stem cells, and engineered natural killer cells. We discuss which cell sources or cell types are promising for clinical applications, such as (i) universal hPSCs or conventional hPSCs and (ii) mature differentiated cells or progenitor cells. Especially, we discuss the progress of clinical trials using hPSC-differentiated cells in this review.
- Open Access
- Review
Overview of Representative Clinical Trials Using Human Pluripotent Stem Cell-Differentiated Cells
Author Information
Received: 19 Nov 2025 | Revised: 17 Dec 2025 | Accepted: 22 Dec 2025 | Published: 26 Dec 2025
Abstract
Graphical Abstract
Keywords
induced pluripotent stem cell-derived cells | embryonic stem cell-derived cells | clinical trial | age-related macular degeneration | progenitor cells
References
- 1.
Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; et al. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147.
- 2.
Takahashi, K.; Tanabe, K.; Ohnuki, M.; et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872.
- 3.
Liu, Y.; Xu, H.W.; Wang, L.; et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 2018, 4, 50.
- 4.
Qiu, T.G. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells (MA09-hRPE) in macular degeneration. NPJ Regen. Med. 2019, 4, 19.
- 5.
Li, S.Y.; Liu, Y.; Wang, L.; et al. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years’ follow-up. Cell Proliferat. 2021, 54, e13100.
- 6.
Takagi, S.; Mandai, M.; Gocho, K.; et al. Evaluation of transplanted autologous induced pluripotent stem cell-derived retinal pigment epithelium in exudative age-related macular degeneration. Ophthalmol. Retina 2019, 3, 850–859.
- 7.
Hirami, Y.; Mandai, M.; Sugita, S.; et al. Safety and stable survival of stem-cell-derived retinal organoid for 2 years in patients with retinitis pigmentosa. Cell Stem Cell 2023, 30, 1585–1596 e1586.
- 8.
da Cruz, L.; Fynes, K.; Georgiadis, O.; et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337.
- 9.
Soomro, T.; Georgiadis, O.; Coffey, P.J.; et al. Safety, structure and function five years after hESC-RPE patch transplantation in acute neovascular AMD with submacular haemorrhage. Graefes. Arch. Clin. Exp. Ophthalmol. 2024, 262, 3057–3060.
- 10.
da Cruz, L.; Soomro, T.; Georgiadis, O.; et al. The fate of RPE cells following hESC-RPE patch transplantation in haemorrhagic Wet AMD: Pigmentation, extension of pigmentation, thickness of transplant, assessment for proliferation and visual function-A 5 year-follow up. Diagnostics 2024, 14, 1005.
- 11.
Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097.
- 12.
Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Transl. Vis. Sci. Technol. 2021, 10, 13.
- 13.
Kashani, A.H.; Lebkowski, J.S.; Hinton, D.R.; et al. Survival of an HLA-mismatched, bioengineered RPE implant in dry age-related macular degeneration. Stem Cell Rep. 2022, 17, 448–458.
- 14.
Humayun, M.S.; Clegg, D.O.; Dayan, M.S.; et al. Long-term follow-up of a phase 1/2a clinical trial of a stem cell-derived bioengineered retinal pigment epithelium implant for geographic atrophy. Ophthalmology 2024, 131, 682–691.
- 15.
Schweitzer, J.S.; Song, B.; Herrington, T.M.; et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 2020, 382, 1926–1932.
- 16.
Kirkeby, A.; Nelander, J.; Hoban, D.B.; et al. Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson’s disease, STEM-PD. Cell Stem Cell 2023, 30, 1299–1314 e1299.
- 17.
Gotkine, M.; Caraco, Y.; Lerner, Y.; et al. Safety and efficacy of first-in-man intrathecal injection of human astrocytes (AstroRx(R)) in ALS patients: Phase I/IIa clinical trial results. J. Transl. Med. 2023, 21, 122.
- 18.
Zhang, H.; Xue, Y.; Pan, T.; et al. Epicardial injection of allogeneic human-induced-pluripotent stem cell-derived cardiomyocytes in patients with advanced heart failure: Protocol for a phase I/IIa dose-escalation clinical trial. BMJ Open 2022, 12, e056264.
- 19.
Miyagawa, S.; Kainuma, S.; Kawamura, T.; et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 950829.
- 20.
Kawamura, T.; Ito, Y.; Ito, E.; et al. Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: First three case reports. Front. Cardiovasc. Med. 2023, 10, 1182209.
- 21.
Menasche, P.; Vanneaux, V.; Hagege, A.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur. Heart J. 2015, 36, 2011–2017.
- 22.
Menasche, P.; Vanneaux, V.; Fabreguettes, J.R.; et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: A translational experience. Eur. Heart J. 2015, 36, 743–750.
- 23.
Menasché, P.; Vanneaux, V.; Hagège, A.; et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438.
- 24.
Shin, J.H.; Ryu, C.M.; Yu, H.Y.; et al. Safety of human embryonic stem cell-derived mesenchymal stem cells for treating interstitial cystitis: A phase I study. Stem Cells Translational Medicine 2022, 11, 1010–1020.
- 25.
Kelly, K.; Bloor, A.J.C.; Griffin, J.E.; et al. Two-year safety outcomes of iPS cell-derived mesenchymal stromal cells in acute steroid-resistant graft-versus-host disease. Nat. Med. 2024, 30, 1556–1558.
- 26.
Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021, 28, 2047–2061 e2045.
- 27.
Shapiro, A.M.J.; Thompson, D.; Donner, T.W.; et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep. Med. 2021, 2, 100466.
- 28.
Keymeulen, B.; De Groot, K.; Jacobs-Tulleneers-Thevissen, D.; et al. Encapsulated stem cell-derived beta cells exert glucose control in patients with type 1 diabetes. Nat. Biotechnol. 2024, 42, 1507–1514.
- 29.
Kirkeby, A.; Main, H.; Carpenter, M. Pluripotent stem-cell-derived therapies in clinical trial: A 2025 update. Cell Stem Cell 2025, 32, 10–37.
- 30.
Wang, X.; Gao, M.; Wang, Y.; The progress of pluripotent stem cell-derived pancreatic beta-cells regeneration for diabetic therapy. Front. Endocrinol. 2022, 13, 927324.
- 31.
Migliorini, A.; Nostro, M.C.; Sneddon, J.B. Human pluripotent stem cell-derived insulin-producing cells: A regenerative medicine perspective. Cell Metab. 2021, 33, 721–731.
- 32.
Ghoneim, M.A.; Gabr, M.M.; El-Halawani, S.M.; et al. Current status of stem cell therapy for type 1 diabetes: A critique and a prospective consideration. Stem Cell Res. Ther. 2024, 15, 23.
- 33.
Wang, G.W.; Heimendinger, P.; Ramelmeier, R.A.; et al. Pluripotent stem cell-based cell therapies: Current applications and future prospects. Curr. Opin. Biomed. Eng. 2022, 22, 100390.
- 34.
Dang, H.P.; Chen, H.; Dargaville, T.R.; et al. Cell delivery systems: Toward the next generation of cell therapies for type 1 diabetes. J. Cell Mol. Med. 2022, 26, 4756–4767.
- 35.
Hogrebe, N.J.; Ishahak, M.; Millman, J.R. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell 2023, 30, 530–548.
- 36.
Kim, J.Y.; Nam, Y.; Rim, Y.A.; et al. Review of the current trends in clinical trials involving induced pluripotent stem cells. Stem Cell Rev. Rep. 2022, 18, 142–154.
- 37.
Yan, L.; Rodriguez-delaRosa, A.; Pourquie, O. Human muscle production in vitro from pluripotent stem cells: Basic and clinical applications. Semin Cell Dev. Biol. 2021, 119, 39–48.
- 38.
Zhang, H.; Jin, Z.B. A rational consideration of the genomic instability of human-induced pluripotent stem cells for clinical applications. Sci. China Life Sci. 2023, 66, 2198–2200.
- 39.
Park, S.J.; Kim, Y.Y.; Han, J.Y.; et al. Advancements in human embryonic stem cell research: Clinical applications and ethical issues. Tissue Eng. Regen. Med. 2024, 21, 379–394.
- 40.
Luce, E.; Messina, A.; Duclos-Vallee, J.C.; et al. Advanced techniques and awaited clinical applications for human pluripotent stem cell differentiation into hepatocytes. Hepatology 2021, 74, 1101–1116.
- 41.
Pendse, S.; Vaidya, A.; Kale, V. Clinical applications of pluripotent stem cells and their derivatives: Current status and future perspectives. Regen. Med. 2022, 17, 677–690.
- 42.
Cong, X.; Zhang, S.M.; Ellis, M.W.; et al. Large animal models for the clinical application of human induced pluripotent stem cells. Stem Cells Dev. 2019, 28, 1288–1298.
- 43.
Kelly, M.I.; Albahrani, M.; Castro, C.; et al. Importance of evaluating protein glycosylation in pluripotent stem cell-derived cardiomyocytes for research and clinical applications. Pflugers Arch. 2021, 473, 1041–1059.
- 44.
Okano, H.; Morimoto, S.; Kato, C.; et al. Induced pluripotent stem cells-based disease modeling, drug screening, clinical trials, and reverse translational research for amyotrophic lateral sclerosis. J. Neurochem. 2023, 167, 603–614.
- 45.
Lam, C.K.; Wu, J.C. Clinical Trial in a Dish: Using patient-derived induced pluripotent stem cells to identify risks of drug-induced cardiotoxicity. Arterioscler Thromb. Vasc. Biol. 2021, 41, 1019–1031.
- 46.
Bretzner, F.; Gilbert, F.; Baylis, F.; et al. Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell 2011, 8, 468–475.
- 47.
Manley, N.C.; Priest, C.A.; Denham, J.; et al. Human embryonic stem cell-derived oligodendrocyte progenitor cells: Preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl. Med. 2017, 6, 1917–1929.
- 48.
Priest, C.A.; Manley, N.C.; Denham, J.; et al. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen. Med. 2015, 10, 939–958.
- 49.
Higuchi, A.; Kumar, S.S.; Benelli, G.; et al. Biomaterials used in stem cell therapy for spinal cord injury. Prog. Mater. Sci. 2019, 103, 374–424.
- 50.
Nakamura, M.; Okano, H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013, 23, 70–80.
- 51.
Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720.
- 52.
Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516.
- 53.
Menasche, P. Cell therapy with human ESC-derived cardiac cells: Clinical perspectives. Front. Bioeng. Biotechnol. 2020, 8, 601560.
- 54.
Bloor, A.J.C.; Patel, A.; Griffin, J.E.; et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nat. Med. 2020, 26, 1720–1725.
- 55.
Nie, W.B.; Zhang, D.; Wang, L.S. Growth factor gene-modified mesenchymal stem cells in tissue regeneration. Drug Des. Devel. Ther. 2020, 14, 1241–1256.
- 56.
Kimbrel, E.A.; Lanza, R. Current status of pluripotent stem cells: Moving the first therapies to the clinic. Nat. Rev. Drug Discov. 2015, 14, 681–692.
- 57.
Carr, A.J.; Smart, M.J.; Ramsden, C.M.; et al. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci. 2013, 36, 385–395.
- 58.
Higuchi, A.; Kumar, S.S.; Benelli, G.; et al. Stem cell therapies for reversing vision loss. Trends Biotechnol. 2017, 35, 1102–1117.
- 59.
Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881.
- 60.
Kim, J.Y.; Zhao, H.; Martinez, J.; et al. Noncanonical autophagy promotes the visual cycle. Cell 2013, 154, 365–376.
- 61.
Mandai, M.; Watanabe, A.; Kurimoto, Y.; et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046.
- 62.
Mehat, M.S.; Sundaram, V.; Ripamonti, C.; et al. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells in macular degeneration. Ophthalmology 2018, 125, 1765–1775.
- 63.
Chen, Q.; Deng, T.; Han, D. Testicular immunoregulation and spermatogenesis. Semin. Cell Dev. Biol. 2016, 59, 157–165.
- 64.
Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3.
- 65.
Taylor, A.W. Ocular immune privilege and transplantation. Front. Immunol. 2016, 7, 37.
- 66.
Benque, I.J.; Xia, P.; Shannon, R.; et al. The neuropeptides of ocular immune privilege, alpha-MSH and NPY, suppress phagosome maturation in macrophages. Immunohorizons 2018, 2, 314–323.
- 67.
Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–671.
- 68.
Rustenhoven, J. A privileged brain. Science 2021, 374, 548.
- 69.
Castellani, G.; Croese, T.; Peralta Ramos, J.M.; et al. Transforming the understanding of brain immunity. Science 2023, 380, eabo7649.
- 70.
Soma, T.; Oie, Y.; Takayanagi, H.; et al. Induced pluripotent stem-cell-derived corneal epithelium for transplant surgery: A single-arm, open-label, first-in-human interventional study in Japan. Lancet 2024, 404, 1929–1939.
- 71.
Silver, S.E.; Barrs, R.W.; Mei, Y. Transplantation of human pluripotent stem cell-derived cardiomyocytes for cardiac regenerative therapy. Front. Cardiovasc. Med. 2021, 8, 707890.
- 72.
Soma, Y.; Morita, Y.; Kishino, Y.; et al. The present state and future perspectives of cardiac regenerative therapy using human pluripotent stem cells. Front. Cardiovasc. Med. 2021, 8, 774389.
- 73.
Bryl, R.; Kulus, M.; Bryja, A.; et al. Cardiac progenitor cell therapy: Mechanisms of action. Cell Biosci. 2024, 14, 30.
- 74.
Okano, S.; Shiba, Y. Therapeutic potential of pluripotent stem cells for cardiac repair after myocardial infarction. Biol. Pharm. Bull. 2019, 42, 524–530.
- 75.
Selvakumar, D.; Clayton, Z.E.; Prowse, A.; et al. Cellular heterogeneity of pluripotent stem cell-derived cardiomyocyte grafts is mechanistically linked to treatable arrhythmias. Nat. Cardiovasc. Res. 2024, 3, 145–165.
- 76.
Nakamura, K.; Neidig, L.E.; Yang, X.; et al. Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes. Stem Cell Rep. 2021, 16, 2473–2487.
- 77.
Shiba, Y.; Gomibuchi, T.; Seto, T.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391.
- 78.
Romagnuolo, R.; Masoudpour, H.; Porta-Sanchez, A.; et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981.
- 79.
Chong, J.J.; Yang, X.; Don, C.W.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277.
- 80.
Tiburcy, M.; Hudson, J.E.; Balfanz, P.; et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 2017, 135, 1832–1847.
- 81.
Schulz, T.C. Concise Review: Manufacturing of pancreatic endoderm cells for clinical trials in type 1 diabetes. Stem Cells Transl. Med. 2015, 4, 927–931.
- 82.
Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439.
- 83.
Rezania, A.; Bruin, J.E.; Arora, P.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133.
- 84.
Mimeault, M.; Batra, S.K. Great promise of tissue-resident adult stem/progenitor cells in transplantation and cancer therapies. Adv. Exp. Med. Biol. 2012, 741, 171–186.
- 85.
Zhao, Z.; Chen, X.; Dowbaj, A.M.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94.
- 86.
Pullen, L.C. Stem cell-derived pancreatic progenitor cells have now been transplanted into patients: Report from IPITA 2018. Am. J. Transplant. 2018, 18, 1581–1582.
- 87.
Bhargava, R.; Mitsides, N.; Saif, I.; et al. C-peptide and combined kidney-pancreas transplantation. NDT Plus 2009, 2, 489–492.
- 88.
Olanow, C.W.; Goetz, C.G.; Kordower, J.H.; et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003, 54, 403–414.
- 89.
Freed, C.R.; Greene, P.E.; Breeze, R.E.; et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001, 344, 710–719.
- 90.
Barker, R.A.; Barrett, J.; Mason, S.L.; et al. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013, 12, 84–91.
- 91.
Piccini, P.; Brooks, D.J.; Bjorklund, A.; et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat. Neurosci. 1999, 2, 1137–1140.
- 92.
Kordower, J.H.; Freeman, T.B.; Snow, B.J.; et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995, 332, 1118–1124.
- 93.
Park, S.; Park, C.W.; Eom, J.H.; et al. Preclinical and dose-ranging assessment of hESC-derived dopaminergic progenitors for a clinical trial on Parkinson’s disease. Cell Stem Cell 2024, 31, 25–38 e28.
- 94.
Tabar, V.; Sarva, H.; Lozano, A. M.; et al. Phase I trial of hES cell-derived dopaminergic neurons for Parkinson's disease. Nature 2025, 641, 978–983.
- 95.
Santos Garcia, D.; De Deus Fonticoba, T.; Paz Gonzalez, J.M.; et al. Staging Parkinson’s disease combining motor and nonmotor symptoms correlates with disability and quality of life. Parkinsons Dis. 2021, 2021, 8871549.
- 96.
Perlmutter, J.S. Assessment of Parkinson disease manifestations. Curr. Protoc. Neurosci. 2009, 10, 10.1.1–10.1.14.
- 97.
Sawamoto, N.; Doi, D.; Nakanishi, E.; et al. Phase I/II trial of iPS-cell-derived dopaminergic cells for Parkinson’s disease. Nature 2025, 641, 971–977.
- 98.
Liu, Q.; Liu, J.; Guo, M.; et al. Comparison of retinal degeneration treatment with four types of different mesenchymal stem cells, human induced pluripotent stem cells and RPE cells in a rat retinal degeneration model. J. Transl. Med. 2023, 21, 910.
- 99.
Han, Y.; Yang, J.; Fang, J.; et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92.
- 100.
Dabrowska, S.; Andrzejewska, A.; Janowski, M.; et al. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: Therapeutic outlook for inflammatory and degenerative diseases. Front. Immunol. 2020, 11, 591065.
- 101.
Trigo, C.M.; Rodrigues, J.S.; Camoes, S.P.; et al. Mesenchymal stem cell secretome for regenerative medicine: Where do we stand? J. Adv. Res. 2025, 70, 103–124.
- 102.
Wang, Y.; Fang, J.; Liu, B.; et al. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530.
- 103.
Margiana, R.; Markov, A.; Zekiy, A.O.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366.
- 104.
Tian, Z.; Wang, C.K.; Lin, F.L.; et al. Effect of extracellular matrix proteins on the differentiation of human pluripotent stem cells into mesenchymal stem cells. J. Mater. Chem. B 2022, 10, 5723–5732.
- 105.
Nosoudi, N.; Hart, C.; McKnight, I.; et al. Differentiation of adipose-derived stem cells to chondrocytes using electrospraying. Sci. Rep. 2021, 11, 24301.
- 106.
Ye, Q.; Sung, T.C.; Yang, J.M.; et al. Generation of universal and hypoimmunogenic human pluripotent stem cells. Cell Prolif. 2020, 53, e12946.
- 107.
Malik, N.N.; Jenkins, A.M.; Mellon, J.; et al. Engineering strategies for generating hypoimmunogenic cells with high clinical and commercial value. Regen. Med. 2019, 14, 983–989.
- 108.
Feucht, J.; Sun, J.; Eyquem, J.; et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 2019, 25, 82–88.
- 109.
Mandal, M.; Clarke, R.; van der Stegen, S.; et al. FT819 path to IND: First-of-kind off-the-shelf CAR19 T-cell for B cell malignancies. Cancer Res. 2020, 80, 3245.
- 110.
Davila, M.L.; Brentjens, R.J. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. 2016, 14, 802–808.
- 111.
Bove, C.; Arcangeli, S.; Falcone, L.; et al. CD4 CAR-T cells targeting CD19 play a key role in exacerbating cytokine release syndrome, while maintaining long-term responses. J. Immunother. Cancer 2023, 11, e005878.
- 112.
Tao, Z.; Chyra, Z.; Kotulova, J.; et al. Impact of T cell characteristics on CAR-T cell therapy in hematological malignancies. Blood Cancer J. 2024, 14, 213.
- 113.
Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69.
- 114.
Mackensen, A.; Muller, F.; Mougiakakos, D.; et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 2022, 28, 2124–2132.
- 115.
Holley, S.M.; Reidling, J.C.; Cepeda, C.; et al. Transplanted human neural stem cells rescue phenotypes in zQ175 Huntington’s disease mice and innervate the striatum. Mol. Ther. 2023, 31, 3545–3563.
- 116.
Reidling, J.C.; Relano-Gines, A.; Holley, S.M.; et al. Human neural stem cell transplantation rescues functional deficits in R6/2 and Q140 Huntington’s disease Mice. Stem Cell Rep. 2018, 10, 58–72.
- 117.
Nicholas, C.R.; Chen, J.; Tang, Y.; et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 2013, 12, 573–586.

This work is licensed under a Creative Commons Attribution 4.0 International License.


