Driven by global resource shortages and carbon neutrality strategies, diatom biorefinery technology has emerged as a key solution to reduce production costs and promote a green economy, thanks to its unique potential for comprehensive resource utilization. Diatoms are rich in high-value substances such as polyunsaturated fatty acids, fucoxanthin, polysaccharides, and phenolic compounds, and their siliceous cell walls hold significant application potential across various industries. However, their industrialization has long been hindered by high production costs, with single-component production models leading to low return on investment—a core bottleneck restricting their development. This review analyzes diatom biorefinery technologies and processes, including co-extraction, sequential extraction, and hierarchical utilization of whole biomass, which significantly enhance resource utilization efficiency and economic benefits. Economic feasibility analyses show that co-producing multiple bioactive substances effectively shares costs, increases profits, and reduces waste treatment expenses, demonstrating promising market prospects. Despite challenges such as upstream process regulation where environmental factors may exert multiple effects on metabolite production, and downstream green chemistry transformation, combining co-production of high-value products with technological innovation can effectively lower costs, achieve efficient resource utilization, and foster sustainable development. Diatom biorefinery processes are poised to achieve larger-scale commercialization, offering new approaches to address global challenges in energy, the environment, and human health.
- Open Access
- Review
Diatom Biorefinery: Comprehensive Resource Utilization and Economic Feasibility for Sustainable Development
- Qinyun Xu 1,
- Keli Yang 2,
- Laodong Guo 3,
- Shikai Wang 1, *
Author Information
Received: 30 May 2025 | Revised: 18 Jun 2025 | Accepted: 24 Jul 2025 | Published: 13 Aug 2025
Abstract
Graphical Abstract
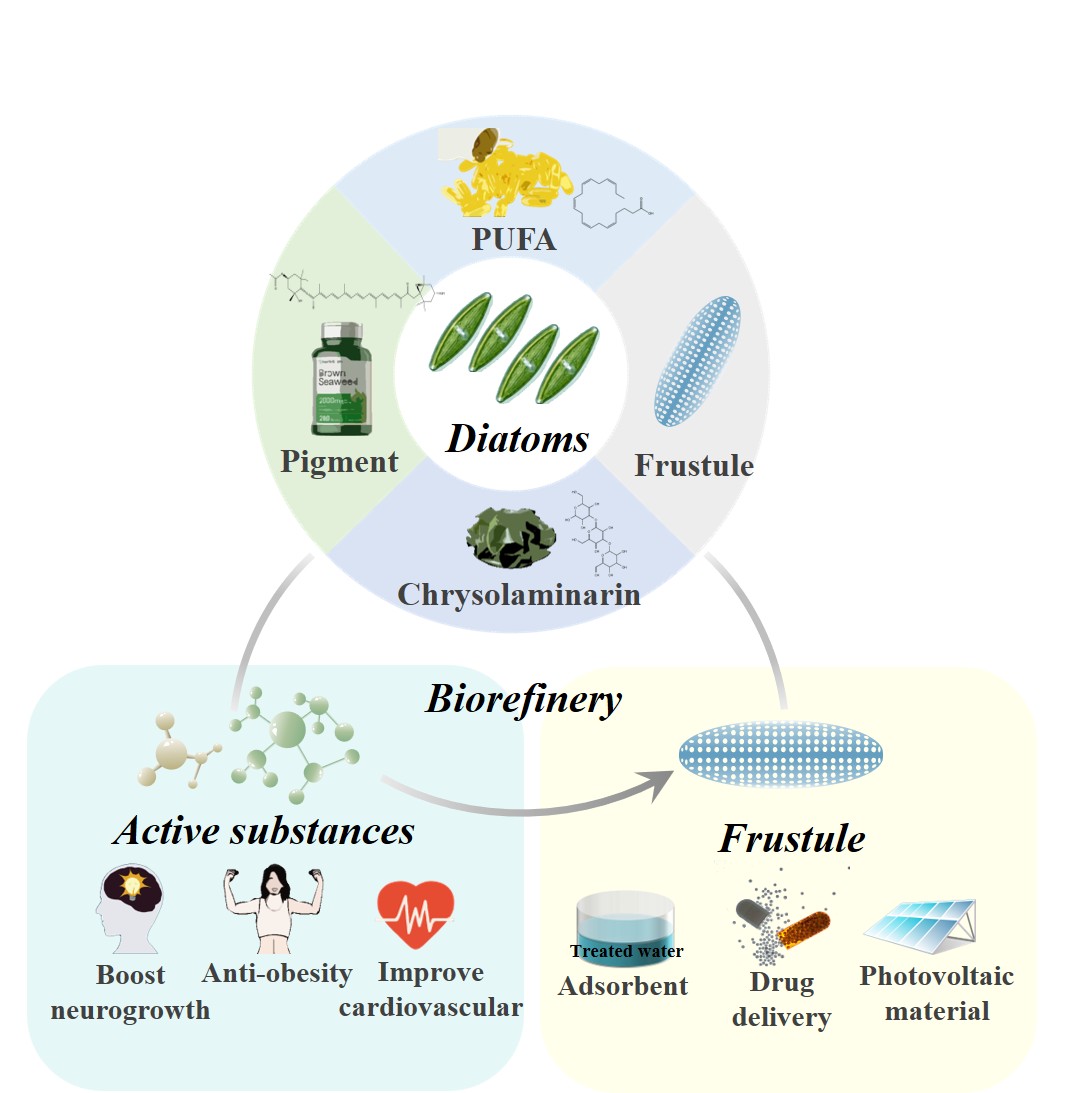
Keywords
diatom | biorefinery | economic analysis | sustainable development
References
- 1.Marella, T.K.; Lopez-Pacheco, I.Y.; Parra-Saldivar, R.; et al. Wealth from waste: Diatoms as tools for phycoremediation of wastewater and for obtaining value from the biomass. Sci. Total Environ. 2020, 724, 137960.
- 2.Mann, D.G.; Vanormelingen, P. An inordinate fondness? The number, distributions, and origins of diatom species. J. Eukaryot. Microbiol. 2013, 60, 414–420.
- 3.Mal, N.; Srivastava, K.; Sharma, Y.; et al. Facets of diatom biology and their potential applications. Biomass Convers. Bior. 2021, 12, 1959–1975.
- 4.Jäger, R.; Heileson, J.L.; Abou Sawan, S.; et al. International society of sports nutrition position stand: Long-chain omega-3 polyunsaturated fatty acids. J. Int. Soc. Sports Nutr. 2025, 22, 2441775.
- 5.Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; et al. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183.
- 6.Gille, A.; Stojnic, B.; Derwenskus, F.; et al. A lipophilic fucoxanthin-rich Phaeodactylum tricornutum extract ameliorates effects of diet-induced obesity in C57BL/6J mice. Nutrients 2019, 11, 796.
- 7.Woo, M. N.; Jeon, S. M.; Shin, Y. C.; et al. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res. 2009, 53, 1603–1611.
- 8.Neumann, U.; Louis, S.; Gille, A.; et al. Anti-inflammatory effects of Phaeodactylum tricornutum extracts on human blood mononuclear cells and murine macrophages. J. Appl. Phycol. 2018, 30, 2837–2846.
- 9.Hosokawa, M.; Kudo, M.; Maeda, H.; et al. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta Gen. Subj. 2004, 1675, 113–119.
- 10.Hosokawa, M.; Wanezaki, S.; Miyauchi, K.; et al. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci. Technol. Res. 1999, 5, 243–246.
- 11.Kim, K.-N.; Heo, S.-J.; Kang, S.-M.; et al. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. In Vitro 2010, 24, 1648–1654.
- 12.Afonso, C.; Bragança, A. R.; Rebelo, B. A.; et al. Optimal nitrate supplementation in Phaeodactylum tricornutum culture medium increases biomass and fucoxanthin production. Foods 2022, 11, 568.
- 13.Medarevic, D.; Losic, D.; Ibric, S. Diatoms—Nature materials with great potential for bioapplications. Hem. Ind. 2016, 70, 613–627.
- 14.Ikusika, O.O.; Mpendulo, C.T.; Zindove, T.J.; et al. Fossil shell flour in livestock production: A Review. Animals 2019, 9, 70.
- 15.Janani, S.; Kumar, S.S. Performance analysis of different textile effluent treatment processes involving marine diatom Odontella aurita. Environ. Technol. Innov. 2018, 11, 153–164.
- 16.Karaman, E.S.; Wang, Z.; Di Benedetto, G.; et al. Fabrication of supercapacitors and flexible electrodes using biosilica from cultured diatoms. Mater. Today Energy 2019, 11, 166–173.
- 17.Bandara, T.M.W.J.; Withanage, S.S.; Wijayaratne, K.B.; et al. Nano structured diatom frustules incorporated into TiO2 photoelectrodes to enhance performance of quasi-solid-state dye-sensitized solar cells. Opt. Mater. 2023, 146, 114514.
- 18.Dolatabadi, J.E.N.; de la Guardia, M. Applications of diatoms and silica nanotechnology in biosensing, drug and gene delivery, and formation of complex metal nanostructures. TrAC-Trend Anal. Chem. 2011, 30, 1538–1548.
- 19.Wang, Y.; Cai, J.; Jiang, Y.; et al. Preparation of biosilica structures from frustules of diatoms and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460.
- 20.Chandrasekaran, S.; Sweetman, M.J.; Kant, K.; et al. Silicon diatom frustules as nanostructured photoelectrodes. Chem. Commun. 2014, 50, 10441–10444.
- 21.Lathifah, W.; Fikri, R.; Hidayati, N.; et al. Effect of commercial NPK fertilizer on growth and biomass of Navicula sp. and Nannochloropsis sp. IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 012060.
- 22.Zhang, H.; Liu, Z.; Chen, J.; et al. Research progress on the production of bioactive substances using marine diatoms. Chin. J. Biotechnol. 2021, 41, 81–90.
- 23.Kumaran, J.; Singh, I.S.B.; Joseph, V. Effective biomass harvesting of marine diatom Chaetoceros muelleri by chitosan-induced flocculation, preservation of biomass, and recycling of culture medium for aquaculture feed application. J. Appl. Phycol. 2021, 33, 1605–1619.
- 24.Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577.
- 25.Farid, M.S.; Shariati, A.; Badakhshan, A.; et al. Using nano-chitosan for harvesting microalga Nannochloropsis sp. Bioresour. Technol. 2013, 131, 555–559.
- 26.Bayu, A.; Rachman, A.; Noerdjito, D.; et al. High-value chemicals from marine diatoms: A biorefinery approach. IOP Conf. Ser. Earth Environ. Sci. 2020, 460, 012012.
- 27.Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192.
- 28.Bedoshvili, Y.D.; Likhoshway, Y.V. Cellular Mechanisms of Diatom Valve Morphogenesis. In Diatoms: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 99–114.
- 29.Anderson, M.W.; Holmes, S.M.; Hanif, N.; et al. Hierarchical pore structures through diatom zeolitization. Angew. Chem. 2000, 39, 2707–2710.
- 30.B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; et al. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia 2022, 850, 2707–2733.
- 31.Cvjetinovic, J.; Luchkin, S. Y.; Statnik, E. S.; et al. Revealing the static and dynamic nanomechanical properties of diatom frustules-Nature's glass lace. Sci. Rep. 2023, 13, 5518.
- 32.Jeffryes, C.; Gutu, T.; Jiao, J.; et al. Metabolic insertion of nanostructured TiO2 into the patterned biosilica of the diatom Pinnularia sp. by a two-stage bioreactor cultivation process. Acs Nano 2008, 2, 2103–2112.
- 33.Davidovich, N.A.; Davidovich, O.I.; Podunai, Y.A.; et al. Reproductive properties of diatoms significant for their cultivation and biotechnology. Russ. J. Plant Physiol. 2015, 62, 153–160.
- 34.Falciatore, A.; Bowler, C. Revealing the molecular secrets of marine diatoms. Annu. Rev. Plant. Biol. 2002, 53, 109–130.
- 35.Guo, B.; Liu, B.; Yang, B.; et al. Screening of diatom strains and characterization of Cyclotella cryptica as a potential fucoxanthin producer. Mar. Drugs 2016, 14, 125.
- 36.Iwasaki, K. Algal Bioproducts: Investigating the Effect of Light Quality on Metabolite Production by Photosynthetic Diatoms. Ph.D. Thesis, University of Technology Sydney, Sydney, NSW, Australia, 2019.
- 37.Mus, F.; Toussaint, J.P.; Cooksey, K.E.; et al. Physiological and molecular analysis of carbon source supplementation and pH stress-induced lipid accumulation in the marine diatom Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2013, 97, 3625–3642.
- 38.Yi, Z.; Su, Y.; Cherek, P.; et al. Combined artificial high-silicate medium and LED illumination promote carotenoid accumulation in the marine diatom Phaeodactylum tricornutum. Microb. Cell Fact. 2019, 18, 209.
- 39.Lee, A.H.; Shin, H.Y.; Park, J.H.; et al. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 543.
- 40.Foo, S.C.; Yusoff, F.M.; Ismail, M.; et al. Production of fucoxanthin-rich fraction (FxRF) from a diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Algal Res. 2015, 12, 26–32.
- 41.Lin, C.-H.; Chang, Y.-F.; Prasetya, S.J.; et al. An integrated process for enhanced production and purification of fucoxanthin and sulfated polysaccharides in diatom Hyalosynedra toxoneides cultures. J. Taiwan. Inst. Chem. Eng. 2024, 155, 105308.
- 42.Li, Y.; Liu, L.; Sun, P.; et al. Fucoxanthinol from the diatom Nitzschia Laevis ameliorates neuroinflammatory responses inlipopolysaccharide-stimulated BV-2 microglia. Mar. Drugs 2020, 18, 116.
- 43.Marella, T.K.; Parine, N.R.; Tiwari, A. Potential of diatom consortium developed by nutrient enrichment for biodiesel production and simultaneous nutrient removal from waste water. Saudi J. Biol. Sci. 2018, 25, 704–709.
- 44.Marella, T.K.; Datta, A.; Patil, M.D.; et al. Biodiesel production through algal cultivation in urban wastewater using algal floway. Bioresour. Technol. 2019, 280, 222–228.
- 45.Pezzolesi, L.; Pichierri, S.; Samorì, C.; et al. PUFAs and PUAs production in three benthic diatoms from the northern Adriatic Sea. Phytochemistry 2017, 142, 85–91.
- 46.Pekkoh, J.; Phinyo, K.; Thurakit, T.; et al. Lipid Profile, antioxidant and antihypertensive activity, and computational molecular docking of diatom fatty acids as ACE inhibitors. Antioxidants 2022, 11, 186.
- 47.Rodolfi, L.; Biondi, N.; Guccione, A.; et al. Oil and eicosapentaenoic acid production by the diatom Phaeodactylum tricornutum cultivated outdoors in Green Wall Panel (GWP®) reactors. Biotechnol. Bioeng. 2017, 114, 2204–2210.
- 48.Zhao, P.; Zang, Z.; Xie, X.; et al. The influence of different flocculants on the physiological activity and fucoxanthin production of Phaeodactylum tricornutum. Process Biochem. 2014, 49, 681–687.
- 49.Świderska-Kołacz, G.; Jefimow, M.; Klusek, J.; et al. Influence of algae supplementation on the concentration of Glutathione and the activity of glutathione enzymes in the mice liver and kidney. Nutrients 2021, 13, 1996.
- 50.Govindan, N.; Maniam, G.P.; Yusoff, M.M.; et al. Statistical optimization of lipid production by the diatom Gyrosigma sp. grown in industrial wastewater. J. Appl. Phycol. 2020, 32, 375–387.
- 51.Kusaikin, M.; Ermakova, S.; Shevchenko, N.; et al. Structural characteristics and antitumor activity of a new chrysolaminaran from the diatom alga Synedra acus. Chem. Nat. Compd. 2010, 46, 1–4.
- 52.Gao, B.; Chen, A.; Zhang, W.; et al. Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. J. Ocean. Univ. China 2017, 16, 916–924.
- 53.Stiefvatter, L.; Neumann, U.; Rings, A.; et al. The microalgae Phaeodactylum tricornutum is well suited as a food with positive effects on the intestinal microbiota and the generation of SCFA: Results from a pre-clinical study. Nutrients 2022, 14, 2504.
- 54.Figueroa, F.A.; Abdala-Díaz, R.; Hernández, V.; et al. Invasive diatom Didymosphenia geminata as a source of polysaccharides with antioxidant and immunomodulatory effects on macrophage cell lines. J. Appl. Phycol. 2019, 32, 93–102.
- 55.Guzman, S.; Gato, A.; Lamela, M.; et al. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670.
- 56.Lee, J.B.; Hayashi, K.; Hirata, M.; et al. Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Biol. Pharm. Bull. 2006, 29, 2135–2139.
- 57.Lakshmegowda, S.B.; Rajesh, S.K.; Kandikattu, H.K.; et al. In vitro and in vivo studies on hexane fraction of Nitzschia palea, a freshwater diatom for oxidative damage protective and anti-inflammatory response. Rev. Bras. Farmacogn. 2020, 30, 189–201.
- 58.Sabia, A.; Clavero, E.; Pancaldi, S.; et al. Effect of different CO2 concentrations on biomass, pigment content, and lipid production of the marine diatom Thalassiosira pseudonana. Appl. Microbiol. Biotechnol. 2018, 102, 1945–1954.
- 59.Chen, G.Q.; Jiang, Y.; Chen, F. Salt-induced alterations in lipid composition of diatom Nitzschia Laevis (Bacillariophyceae) under heterotrophic culture condition. J. Phycol. 2008, 44, 1309–1314.
- 60.Cheng, J.; Feng, J.; Ge, T.; et al. Pyrolytic characteristics of biodiesel prepared from lipids accumulated in diatom cells with growth regulation. J. Biosci. Bioeng. 2015, 120, 161–166.
- 61.Lin, Q.; Zhuo, W.H.; Wang, X.W.; et al. Effects of fundamental nutrient stresses on the lipid accumulation profiles in two diatom species Thalassiosira weissflogii and Chaetoceros muelleri. Bioprocess Biosyst. Eng. 2018, 41, 1213–1224.
- 62.Svenning, J.B.; Dalheim, L.; Vasskog, T.; et al. Lipid yield from the diatom Porosira glacialis is determined by solvent choice and number of extractions, independent of cell disruption. Sci. Rep. 2020, 10, 22229.
- 63.Sabharwal, T.; Sathasivan, K.; Mehdy, M.C. Defense related decadienal elicits membrane lipid remodeling in the diatom Phaeodactylum tricornutum. PLoS ONE 2017, 12, e0178761.
- 64.Shishlyannikov, S.M.; Klimenkov, I.V.; Bedoshvili, Y.D.; et al. Effect of mixotrophic growth on the ultrastructure and fatty acid composition of the diatom Synedra acus from Lake Baikal. J. Biol. Res. Thessalon. 2014, 21, 15.
- 65.Artamonova, E.Y.; Svenning, J.B.; Vasskog, T.; et al. Analysis of phospholipids and neutral lipids in three common northern cold water diatoms: Coscinodiscus concinnus, Porosira glacialis, and Chaetoceros socialis, by ultra-high performance liquid chromatography-mass spectrometry. J. Appl. Phycol. 2017, 29, 1241–1249.
- 66.Scholz, B.; Liebezeit, G. Biochemical characterisation and fatty acid profiles of 25 benthic marine diatoms isolated from the Solthörn tidal flat (southern North Sea). J. Appl. Phycol. 2012, 25, 453–465.
- 67.Dalheim, L.; Svenning, J.B.; Eilertsen, H.C.; et al. Stability of lipids during wet storage of the marine diatom Porosira glacialis under semi-preserved conditions at 4 and 20 °C. J. Appl. Phycol. 2020, 33, 385–395.
- 68.Govindan, N.; Maniam, G.; Ab. Rahim, M.; et al. Production of renewable lipids by the diatom Amphora copulata. Fermentation 2021, 7, 37.
- 69.Cointet, E.; Séverin, E.; Couzinet-Mossion, A.; et al. Assessment of the lipid production potential of six benthic diatom species grown in airlift photobioreactors. J. Appl. Phycol. 2021, 33, 2093–2103.
- 70.Palanisamy, K.M.; Paramasivam, P.; Maniam, G.P.; et al. Production of lipids by Chaetoceros affinis in media based on palm oil mill effluent. J. Biotechnol. 2021, 327, 86–96.
- 71.Zhukova, N.; Aizdaicher, N. Lipid and fatty acid composition during vegetative and resting stages of the marine diatom Chaetoceros salsugineus. Bot. Mar. 2001, 44, 287–293.
- 72.Liang, Y.; Mai, K. Effect of growth phase on the fatty acid compositions of four species of marine diatoms. J. Ocean. Univ. China 2005, 4, 157–162.
- 73.Yang, Y.H.; Du, L.; Hosokawa, M.; et al. Fatty acid and lipid class composition of the microalga Phaeodactylum tricornutum. J. Oleo Sci. 2017, 66, 363–368.
- 74.Chen, Y.-C. The biomass and total lipid content and composition of twelve species of marine diatoms cultured under various environments. Food Chem. 2012, 131, 211–219.
- 75.Saranya, G.; Ramachandra, T.V. Scope for biodiesel and bioactive compounds production in the diatom Nitzschia punctata. Fuel 2021, 300, 120985.
- 76.Krishnan, A.; Anandan, R.; Joseph, A. Culture medium and growth phase modulate the fatty acid composition of the diatom Nitzschia palea (Kutzing) W. Smith-Potential source for live feed and biodiesel. Fish. Technol. 2020, 57, 28–35.
- 77.Bouzidi, N.; Zili, F.; García-Maroto, F.; et al. Impact of temperature and growth phases on lipid composition and fatty acid profile of a thermophilic Bacillariophyta strain related to the genus Halamphora from north-eastern Tunisia. J. Mar. Biol. Assoc. UK 2020, 100, 529–536.
- 78.Bedoshvili, Y.; Podunay, Y.; Nikonova, A.; et al. Lipid and fatty acids accumulation features of Entomoneis cf. paludosa during exponential and stationary growth phases in laboratory culture. Diversity 2021, 13, 459.
- 79.Demirel, Z.; Imamoglu, E.; Dalay, M.C. Growth kinetics of Nanofrustulum shiloi under different mixing conditions in flat-plate photobioreactor. Braz. Arch. Biol. Technol. 2020, 63, e20190201.
- 80.Ying, L.; Kang-sen, M.; Shi-chun, S.; et al. Effect of light intensity on the total lipid and fatty acid composition of six strains of marine diatoms. Chin. J. Oceanol. Limnol. 2001, 19, 249–254.
- 81.Wang, X.W.; Liang, J.R.; Luo, C.S.; et al. Biomass, total lipid production, and fatty acid composition of the marine diatom Chaetoceros muelleri in response to different CO2 levels. Bioresour. Technol. 2014, 161, 124–130.
- 82.Niu, Y.-F.; Wang, X.; Hu, D.-X.; et al. Molecular characterization of a glycerol-3-phosphate acyltransferase reveals key features essential for triacylglycerol production in Phaeodactylum tricornutum. Biotechnol. Biofuels 2016, 9, 60.
- 83.Cheah, Y.T.; Ng, B.W.; Tan, T.L.; et al. Biomass and eicosapentaenoic acid production from Amphora sp. under different environmental and nutritional conditions. Biotechnol. Appl. Biochem. 2023, 70, 568–580.
- 84.Levitan, O.; Dinamarca, J.; Hochman, G.; et al. Diatoms: A fossil fuel of the future. Trends Biotechnol. 2014, 32, 117–124.
- 85.Sahena, F.; Zaidul, I.S.M.; Jinap, S.; et al. Fatty acid compositions of fish oil extracted from different parts of Indian mackerel (Rastrelliger kanagurta) using various techniques of supercritical CO2 extraction. Food Chem. 2010, 120, 879–885.
- 86.Yang, M.; Wei, B.; Meng, J.; et al. Sources and Physiological Functions of ω-3 Polyunsaturated Fatty Acids: A Research Progress. China Oils Fats 2019, 44, 110–115.
- 87.Adarme-Vega, T.C.; Thomas-Hall, S.R.; Schenk, P.M. Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 2014, 26, 14–18.
- 88.Peltomaa, E.; Johnson, M.D.; Taipale, S.J. Marine cryptophytes are great sources of EPA and DHA. Mar. Drugs 2017, 16, 3.
- 89.Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7.
- 90.Brenes-Monge, H.P.; del Pilar Sánchez-Saavedra, M. Effect of nitrogen limitation and irradiance on the biochemical composition of Haslea ostrearia. Algal Res. 2025, 86, 103931.
- 91.Torstensson, A.; Hedblom, M.; Andersson, J.; et al. Synergism between elevated pCO2 and temperature on the Antarctic sea ice diatom Nitzschia lecointei. Biogeosciences 2013, 10, 6391–6401.
- 92.Tyagi, R.; Singh, P.K.; Saxena, A.; et al. Exploring the nutraceutical potential of high-altitude freshwater diatom Nitzschia sp. in batch culture. Syst. Microbiol. Biomanuf. 2024, 4, 1262–1272.
- 93.de Viçose, G.C.; Porta, A.; Viera, M.P.; et al. Effects of density on growth rates of four benthic diatoms and variations in biochemical composition associated with growth phase. J. Appl. Phycol. 2012, 24, 1427–1437.
- 94.Guihéneuf, F.; Fouqueray, M.; Mimouni, V.; et al. Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae Pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J. Appl. Phycol. 2010, 22, 629–638.
- 95.Pasquet, V.; Ulmann, L.; Mimouni, V.; et al. Fatty acids profile and temperature in the cultured marine diatom Odontella aurita. J. Appl. Phycol. 2014, 26, 2265–2271.
- 96.Hamilton, M.L.; Warwick, J.; Terry, A.; et al. Towards the industrial production of omega-3 long chain polyunsaturated fatty acids from a genetically modified diatom Phaeodactylum tricornutum. PLoS ONE 2015, 10, e0144054.
- 97.Qiao, H.; Cong, C.; Sun, C.; et al. Effect of culture conditions on growth, fatty acid composition and DHA/EPA ratio of Phaeodactylum tricornutum. Aquaculture 2016, 452, 311–317.
- 98.Şirin, P.A.; Serdar, S. Effects of nitrogen starvation on growth and biochemical composition of some microalgae species. Folia Microbiol. 2024, 69, 889–902.
- 99.Steinrucken, P.; Prestegard, S.K.; de Vree, J.H.; et al. Comparing EPA production and fatty acid profiles of three Phaeodactylum tricornutum strains under western Norwegian climate conditions. Algal Res. 2018, 30, 11–22.
- 100.Ruiz-Domínguez, M.C.; Toledo, C.; Órdenes, D.; et al. Variability of omega-3/6 fatty acid obtained through extraction-transesterification processes from Phaeodactylum tricornutum. Acta Chim. Slov. 2021, 68, 629–637.
- 101.Svenning, J.B.; Dalheim, L.; Eilertsen, H.C.; et al. Temperature dependent growth rate, lipid content and fatty acid composition of the marine cold-water diatom Porosira glacialis. Algal Res. 2019, 37, 11–16.
- 102.Artamonova, E.Y.; Vasskog, T.; Eilertsen, H.C. Lipid content and fatty acid composition of Porosira glacialis and Attheya longicornis in response to carbon dioxide (CO2) aeration. PLoS ONE 2017, 12, e0177703.
- 103.Bastos, C.R.V.; Maia, I.B.; Pereira, H.; et al. Optimisation of biomass production and nutritional value of two marine diatoms (Bacillariophyceae), Skeletonema costatum and Chaetoceros calcitrans. Biology 2022, 11, 594.
- 104.Wu, M.; Gao, G.; Jian, Y.; et al. High CO2 increases lipid and polyunsaturated fatty acid productivity of the marine diatom Skeletonema costatum in a two-stage model. J. Appl. Phycol. 2022, 34, 43–50.
- 105.Gao, X.Z.; Jiang, X.M.; Zhang, Z.L.; et al. Comparative study on total lipid content and fatty acid composition of five newly isolated marine diatoms. J. Biol. 2014, 31, 60–63, 81.
- 106.Zhukova, N. Changes in the lipid composition of Thalassiosira pseudonana during its life cycle. Russ. J. Plant Physiol. 2004, 51, 702–707.
- 107.Etesami, E.; Jorjani, S.; Noroozi, M. Improvement of Thalassiosira weissflogii as high valuable nutritional feed. Iran. J. Fish. Sci. 2022, 21, 15–32.
- 108.Suroy, M.; Moriceau, B.; Boutorh, J.; et al. Fatty acids associated with the frustules of diatoms and their fate during degradation—A case study in Thalassiosira weissflogii. Deep. Sea Res. Part. I Oceanogr. Res. Pap. 2014, 86, 21–31.
- 109.Ofosu, F.K.; Daliri, E.B.; Lee, B.H.; et al. Current trends and future perspectives on omega-3 fatty acids. Res. Rev. J. Biol. 2017, 5, 11–20.
- 110.Tyagi, R.; Rastogi, R.P.; Babich, O.; et al. New perspectives of omega-3 fatty acids from diatoms. Syst. Microbiol. Biomanuf. 2023, 4, 528–541.
- 111.Khozin-Goldberg, I.; Sayanova, O. Metabolic engineering and synthetic biology approaches to enhancing production of long-chain polyunsaturated fatty acids in microalgae. In Grand Challenges in Algae Biotechnology. Grand Challenges in Biology and Biotechnology; Hallmann, A., Rampelotto, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 249–289.
- 112.Arao, T.; Yamada, M. Biosynthesis of polyunsaturated fatty acids in the marine diatom, Phaeodactylum tricornutum. Phytochemistry 1994, 35, 1177–1181.
- 113.Gong, Y.; Wan, X.; Jiang, M.; et al. Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Prog. Lipid Res. 2014, 56, 19–35.
- 114.Metherel, A.H.; Bazinet, R.P. Updates to the n-3 polyunsaturated fatty acid biosynthesis pathway: DHA synthesis rates, tetracosahexaenoic acid and (minimal) retroconversion. Prog. Lipid Res. 2019, 76, 101008.
- 115.Meyer, A.; Cirpus, P.; Ott, C.; et al. Biosynthesis of docosahexaenoic acid in Euglena gracilis: Biochemical and molecular evidence for the involvement of a Δ4-fatty acyl group desaturase. Biochemistry 2003, 42, 9779–9788.
- 116.Jones, P.J.; Papamandjaris, A.A. Lipids: Cellular metabolism. In Present knowledge in nutrition; Wiley: Hoboken, NJ, USA, 2012; 132–148.
- 117.Akiba, S.; Murata, T.; Kitatani, K.; et al. Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol. Pharm. Bull. 2000, 23, 1293–1297.
- 118.Tapiero, H.; Ba, G.N.; Couvreur, P.; et al. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222.
- 119.De Lau, L.; Bornebroek, M.; Witteman, J.; et al. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam study. Neurology 2005, 64, 2040–2045.
- 120.Julien, C.; Berthiaume, L.; Hadj-Tahar, A.; et al. Postmortem brain fatty acid profile of levodopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem. Int. 2006, 48, 404–414.
- 121.Morris, M.C.; Evans, D.A.; Bienias, J.L.; et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946.
- 122.Wang, C.; Wang, D.; Xu, J.; et al. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson’s disease. J. Funct. Foods 2018, 45, 417–426.
- 123.Calon, F.; Lim, G.P.; Yang, F.; et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron 2004, 43, 633–645.
- 124.Garg, P.; Pejaver, R.K.; Sukhija, M.; et al. Role of DHA, ARA, & phospholipids in brain development: An Indian perspective. Clin. Epidemiol. Glob. Health 2017, 5, 155–162.
- 125.Zhai, S.D.; Jiang, L.Q.; Liu, F. New advances in clinical applications of Ω-3 unsaturated fatty acids. Chin. J. New Drugs 2003, 2, 98–101.
- 126.Kesavulu, M.M.; Kameswararao, B.; Apparao, C.; et al. Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab. 2002, 28, 20–26.
- 127.Pajot, A.; Hao Huynh, G.; Picot, L.; et al. Fucoxanthin from algae to human, an extraordinary bioresource: Insights and advances in up and downstream processes. Mar. Drugs 2022, 20, 222.
- 128.Xia, S.; Wang, K.; Wan, L.; et al. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681.
- 129.Fang, J.P.; Chen, Q.C.; Huang, L.Q. Research progress on the biosynthetic pathway of fucoxanthin and its response to light. J. Fujian Norm. Univ. 2021, 37, 96–108.
- 130.Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; et al. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483.
- 131.Fernandes, F.; Barbosa, M.; Oliveira, A.P.; et al. The pigments of kelps (Ochrophyta) as part of the flexible response to highly variable marine environments. J. Appl. Phycol. 2016, 28, 3689–3696.
- 132.Oliyaei, N.; Moosavi-Nasab, M. Ultrasound-assisted extraction of fucoxanthin from Sargassum angustifolium and Cystoseira indica brown algae. J. Food Process. Preserv. 2021, 45, e15929.
- 133.Molina, G.A.; González-Reyna, M.A.; Loske, A.M.; et al. Weak shock wave-mediated fucoxanthin extraction from Sargassum spp. and its electrochemical quantification. Algal Res. 2022, 68, 102891.
- 134.Jaswir, I.; Noviendri, D.; Salleh, H.M.; Miyashita, K. Fucoxanthin extractions of brown seaweeds and analysis of their lipid fraction in methanol. Food Sci. Technol. Res. 2012, 18, 251–257.
- 135.Savira, A.D.R.; Amin, M.N.G.; Alamsjah, M.A. The effect of different type of solvents on the antioxidant activity of fucoxanthin extract from brown seaweed Sargassum duplicatum. IOP Conf. Ser. Earth Environ. Sci. 2021, 718, 012010.
- 136.Ktari, L.; Mdallel, C.; Aoun, B.; et al. Fucoxanthin and phenolic contents of six dictyotales from the tunisian coasts with an emphasis for a green extraction using a supercritical CO2 method. Front. Mar. Sci. 2021, 8, 647159.
- 137.Xiao, X.; Si, X.; Yuan, Z.; et al. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317.
- 138.Nunes, N.; Leça, J.M.; Pereira, A.C.; et al. Evaluation of fucoxanthin contents in seaweed biomass by vortex-assisted solid-liquid microextraction using high-performance liquid chromatography with photodiode array detection. Algal Res. 2019, 42, 101603.
- 139.Jaswir, I.; Noviendri, D.; Salleh, H.M.; et al. Analysis of fucoxanthin content and purification of all-trans-fucoxanthin from Turbinaria turbinata and Sargassum plagyophyllum by SiO2 open column chromatography and reversed phase-HPLC. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1340–1354.
- 140.Kanda, H.; Kamo, Y.; Machmudah, S.; et al. Extraction of fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar. Drugs 2014, 12, 2383–2396.
- 141.Shannon, E.; Abu-Ghannam, N. Optimisation of fucoxanthin extraction from Irish seaweeds by response surface methodology. J. Appl. Phycol. 2016, 29, 1027–1036.
- 142.Raji, V.; Loganathan, C.; Sadhasivam, G.; et al. Purification of fucoxanthin from Sargassum wightii Greville and understanding the inhibition of angiotensin 1-converting enzyme: An in vitro and in silico studies. Int. J. Biol. Macromol. 2020, 148, 696–703.
- 143.Shang, Y.F.; Kim, S.M.; Lee, W.J.; et al. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J. Biosci. Bioeng. 2011, 111, 237–241.
- 144.Ye, Y.; Sun, J.; Wang, L.; et al. Isolation and purification of fucoxanthin from brown seaweed Sargassum horneri using open ODS column chromatography and ethanol precipitation. Molecules 2021, 26, 3777.
- 145.McClure, D.D.; Luiz, A.; Gerber, B.; et al. An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48.
- 146.Derwenskus, F.; Metz, F.; Gille, A.; et al. Pressurized extraction of unsaturated fatty acids and carotenoids from wet Chlorella vulgaris and Phaeodactylum tricornutum biomass using subcritical liquids. GCB Bioenergy 2018, 11, 335–344.
- 147.Khoo, K.S.; Ooi, C.W.; Chew, K.W.; et al. Extraction of fucoxanthin from Chaetoceros calcitrans by electropermeabilization-assisted liquid biphasic flotation system. J. Chromatogr. A 2022, 1668, 462915.
- 148.Eilers, U.; Bikoulis, A.; Breitenbach, J.; et al. Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. J. Appl. Phycol. 2015, 28, 123–129.
- 149.Paidi, M.K.; Attupuram, A.; Udata, K.S.; et al. Acetone diethyl ether-based biorefinery process for co-extraction of fucoxanthin, chlorophyll, DHA, and EPA from the diatom Thalassiosira lundiana. Algal Res. 2023, 74, 103215.
- 150.Popovich, C.A.; Faraoni, M.B.; Sequeira, A.; Det al. Potential of the marine diatom Halamphora coffeaeformis to simultaneously produce omega-3 fatty acids, chrysolaminarin and fucoxanthin in a raceway pond. Algal Res. 2020, 51, 102030.
- 151.Lu, X.; Liu, B.; He, Y.; et al. Novel insights into mixotrophic cultivation of Nitzschia laevis for co-production of fucoxanthin and eicosapentaenoic acid. Bioresour. Technol. .2019, 294, 122145.
- 152.Kim, S.M.; Jung, Y.J.; Kwon, O.N.; et al. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855.
- 153.Gilbert-López, B.; Barranco, A.; Herrero, M.; et al. Development of new green processes for the recovery of bioactives from Phaeodactylum tricornutum. Food Res. Int. 2017, 99, 1056–1065.
- 154.Wang, S.; Verma, S.K.; Hakeem Said, I.; et al. Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light. Microb. Cell Fact. 2018, 17, 1–13.
- 155.Khoo, K.S.; Ooi, C.W.; Chew, K.W.; et al. Bioprocessing of Chaetoceros calcitrans for the recovery of fucoxanthin using CO2-based alkyl carbamate ionic liquids. Bioresour. Technol. 2021, 322, 124520.
- 156.Sun, J.; Zhou, C.; Cheng, P.; et al. A simple and efficient strategy for fucoxanthin extraction from the microalga Phaeodactylum tricornutum. Algal Res. 2022, 61, 102610.
- 157.Xia, S.; Gao, B.; Fu, J.; et al. Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. J. Biosci. Bioeng. 2018, 126, 723–729.
- 158.Marella, T.K.; Tiwari, A. Marine diatom Thalassiosira weissflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour. Technol. 2020, 307, 123245.
- 159.Tachihana, S.; Nagao, N.; Katayama, T.; et al. High productivity of eicosapentaenoic acid and fucoxanthin by a marine diatom Chaetoceros gracilis in a semi-continuous culture. Front. Bioeng. Biotechnol. 2020, 8, 602721.
- 160.Yang, R.; Wei, D.; Xie, J. Diatoms as cell factories for high-value products: Chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009.
- 161.Seo, M.J.; Seo, Y.J.; Pan, C.H.; et al. Fucoxanthin suppresses lipid accumulation and ROS production during differentiation in 3T3-L1 adipocytes. Phytother. Res. 2016, 30, 1802–1808.
- 162.Maria, A.G.; Graziano, R.; Nicolantonio, D.O. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762.
- 163.Maeda, H.; Hosokawa, M.; Sashima, T.; et al. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397.
- 164.Mei, C.; Zhou, S.; Zhu, L.; et al. Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar. Drugs 2017, 15, 39.
- 165.Ye, G.; Wang, L.; Yang, K.; et al. Fucoxanthin may inhibit cervical cancer cell proliferation via downregulation of HIST1H3D. J. Int. Med. Res. 2020, 48, 1–14.
- 166.Zhu, Y.; Cheng, J.; Min, Z.; et al. Effects of fucoxanthin on autophagy and apoptosis in SGC-7901cells and the mechanism. J. Cell. Biochem. 2018, 119, 7274–7284.
- 167.Wang, J.; Ma, Y.; Yang, J.; et al. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229.
- 168.Zhang, Y.; Fang, H.; Xie, Q.; et al. Comparative evaluation of the radical-scavenging activities of fucoxanthin and its stereoisomers. Molecules 2014, 19, 2100–2113.
- 169.Raji, V.; Loganathan, C.; Ramesh, T.; et al. Dual antidiabetic and antihypertensive activity of fucoxanthin isolated from Sargassum wightii Greville in in vivo rat model. Food Sci. Hum. Wellness 2023, 12, 1693–1700.
- 170.Xu, H.Y.; Jiang, M.T.; Yang, Y.F.; et al. Microalgae-based fucoxanthin attenuates rheumatoid arthritis by targeting the JAK-STAT signaling pathway and gut microbiota. J. Agric. Food Chem. 2025, 73, 11708–11719.
- 171.Zhang, L.; Li, T.; Liu, J.; et al. The regulation of the NF-κB p65 and Nrf2/HO-1 signaling pathways by fucoxanthin in human THP-1 monocyte macrophages under a lipopolysaccharide-induced inflammation model. Foods 2025, 14, 1746.
- 172.Zhou, Y.; Zhang, J.; Xu, K.; et al. Fucoxanthin improves serum lipids, liver metabolism and gut microbiota in hyperlipidemia mice. Food Sci. Hum. Wellness 2025, 14, 9250017.
- 173.Kroth, P.G.; Chiovitti, A.; Gruber, A.; et al. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS ONE 2008, 3, e1426.
- 174.Gügi, B.; Le Costaouec, T.; Burel, C.; et al. Diatom-specific oligosaccharide and polysaccharide structures help to unravel biosynthetic capabilities in diatoms. Mar. Drugs 2015, 13, 5993–6018.
- 175.Ben Atitallah, A.; Hentati, F.; Dammak, M.; et al. Effect of microalgae incorporation on quality characteristics and functional and antioxidant capacities of ready-to-eat fish burgers made from common carp (Cyprinus carpio). Appl. Sci. 2019, 9, 1830.
- 176.Tiwari, A.; Melchor-Martínez, E.M.; Saxena, A.; et al. Therapeutic attributes and applied aspects of biological macromolecules (polypeptides, fucoxanthin, sterols, fatty acids, polysaccharides, and polyphenols) from diatoms—A review. Int. J. Biol. Macromol. 2021, 171, 398–413.
- 177.Qin, J.; Wang, J.-K.; Zhang, J.-T. Adsorption properties of diatom frustules for heavy metal Cu2+. Guangdong Chem. Ind. 2016, 43, 93–95.
- 178.Phogat, S.; Saxena, A.; Kapoor, N.; et al. Diatom mediated smart drug delivery system. J. Drug Deliv. Sci. Technol. 2021, 63, 102433.
- 179.Aw, M.S.; Simovic, S.; Addai-Mensah, J.; et al. Silica microcapsules from diatoms as new carrier for delivery of therapeutics. Nanomedicine 2011, 6, 1159–1173.
- 180.Jeffryes, C.; Campbell, J.; Li, H.; et al. The potential of diatom nanobiotechnology for applications in solar cells, batteries, and electroluminescent devices. Energy Environ. Sci. 2011, 4, 3930–3941.
- 181.Bandara, T.; Furlani, M.; Albinsson, I.; et al. Diatom frustules enhancing the efficiency of gel polymer electrolyte based dye-sensitized solar cells with multilayer photoelectrodes. Nanoscale Adv. 2020, 2, 199–209.
- 182.Gautam, S.; Kashyap, M.; Gupta, S.; et al. Metabolic engineering of TiO2 nanoparticles in Nitzschia palea to form diatom nanotubes: An ingredient for solar cells to produce electricity and biofuel. RSC advances 2016, 6, 97276–97284.
- 183.Sun, X.W.; Zhang, Y.X.; Losic, D. Diatom silica, an emerging biomaterial for energy conversion and storage. J. Mater. Chem. A 2017, 5, 8847–8859.
- 184.Huang, D.-R.; Jiang, Y.-J.; Liou, R.-L.; et al. Enhancing the efficiency of dye-sensitized solar cells by adding diatom frustules into TiO2 working electrodes. Appl. Surf. Sci. 2015, 347, 64–72.
- 185.Tan, T.-W.; Yu, J.-L.; Zhang, X. Recent advances in biorefinery technology research. Chem. Eng. Prog. 2011, 30, 117–125.
- 186.Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145.
- 187.Li, M.; Zou, W.; Kou, H.; et al. Research progress on biorefining of sorghum straw. Food Ferment. Ind. 2023, 49, 358–366.
- 188.Kholany, M.; Coutinho, J.A.P.; Ventura, S.P.M. Carotenoid production from microalgae: The portuguese scenario. Molecules 2022, 27, 2540.
- 189.Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; et al. Large-scale production of Spirulina-based proteins and c-phycocyanin: A biorefinery approach. Biochem. Eng. J. 2022, 185, 108541.
- 190.Mussagy, C.U.; Caicedo-Paz, A.V.; Figueroa, D.; et al. Maximizing Haematococcus biorefineries: Ionic liquid-based astaxanthin recovery, biocosmetic formulation, solar cell applications, and biofertilizer valorization. Bioresour. Technol. 2025, 426, 132347.
- 191.Delbrut, A.; Albina, P.; Lapierre, T.; et al. Fucoxanthin and polyunsaturated fatty acids co-extraction by a green process. Molecules 2018, 23, 874.
- 192.Zhang, W.; Wang, F.; Gao, B.; et al. An integrated biorefinery process: Stepwise extraction of fucoxanthin, eicosapentaenoic acid and chrysolaminarin from the same Phaeodactylum tricornutum biomass. Algal Res. 2018, 32, 193–200.
- 193.YH Research. Food-Grade Fucoxanthin Market Analysis. Available online: https://www.yhresearch.cn/reports/2155476/food-grade-fucoxanthin (accessed on 13 January 2025).
- 194.YH Research. Omega-3 PUFA Market Research Report. Available online: https://www.yhresearch.cn/reports/1338705/omega-3-pufa (accessed on 25 December 2023).
- 195.YH Research. Global and China Diatomite Industry Top Enterprise Market Share and Ranking Research Report in 2025. Available online: https://www.yhresearch.cn/reports/2113867/diatomaceous-earth (accessed on 19 January 2025).
- 196.Diatomite Market Size and Share Outlook—Forecast Trends and Growth Analysis Report (2025–2034) Available online: https://www.expertmarketresearch.com/reports/diatomite-market (accessed on 1 April 2025).
- 197.Gilcher, E.B.; Lane, M.K.M.; Pontious, R.S.; et al. Sequential extraction and purification of triglycerides and carotenoids with supercritical carbon dioxide for valorization of the integrated algal biorefinery. ACS. Sustain. Chem. Eng. 2025, 13, 1667–1676.
- 198.Weickert, S.; Schmid-Staiger, U.; Lewandowski, I. Influence of specific light availability and solvent on process economics—The production of fucoxanthin and eicosapentaenoic acid from P. tricornutum using flat-panel airlift photobioreactors with artificial light. Algal Res. 2023, 75, 103284.
- 199.Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; et al. Microalgal biorefinery concepts’ developments for biofuel and bioproducts: Current perspective and bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623.
- 200.Chew, K.W.; Chia, S.R.; Show, P.L.; et al. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan. Inst. Chem. Eng. 2018, 91, 332–344.
- 201.Zhao, Y.; Sun, Y.; Zhu, Z.; et al. Effects of salinity and temperature on growth performance, biochemical composition, and biosilification process of Cyclotella cryptica. Algal Res. 2024, 84, 103751.
- 202.Yi, Z.; Xu, M.; Magnusdottir, M.; et al. Photo-oxidative stress-driven mutagenesis and adaptive evolution on the marine diatom Phaeodactylum tricornutum for enhanced carotenoid accumulation. Mar. Drugs 2015, 13, 6138–6151.
- 203.Wang, S.; Wu, S.; Yang, G.; et al. A review on the progress, challenges and prospects in commercializing microalgal fucoxanthin. Biotechnol. Adv. 2021, 53, 107865.
- 204.Pocha, C.K.R.; Chia, W.Y.; Chew, K.W.; et al. Current advances in recovery and biorefinery of fucoxanthin from Phaeodactylum tricornutum. Algal Res. 2022, 65, 102735.
- 205.Bozarth, A.; Maier, U.-G.; Zauner, S. Diatoms in biotechnology: Modern tools and applications. Appl. Microbiol. Biotechnol. 2009, 82, 195–201.
- 206.Pang, Y.; Duan, L.; Song, B.; et al. A Review of fucoxanthin biomanufacturing from Phaeodactylum tricornutum. Bioprocess Biosyst. Eng. 2024, 47, 1951–1972.
- 207.Budiarso, F.S.; Leong, Y.K.; Chang, J.-J.; et al. Current advances in microalgae-based fucoxanthin production and downstream processes. Bioresour. Technol. 2025, 428, 132455.
- 208.Akyıl, S.; İlter, I.; Koç, M.; et al. Effects of extraction methods and conditions on bioactive compounds extracted from Phaeodactylum tricornutum. Acta Chim. Slov. 2020, 67, 1250–1261.
How to Cite
Xu, Q.; Yang, K.; Guo, L.; Wang, S. Diatom Biorefinery: Comprehensive Resource Utilization and Economic Feasibility for Sustainable Development. Sustainable Engineering Novit 2025, 1 (1), 3. https://doi.org/10.53941/sen.2025.100003.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


