This review explores the transformative role of multiscale modeling in advancing fuel cell and electrolyzer technologies, which are essential for achieving decarbonized energy systems. By integrating quantum-level reaction mechanisms, mesoscale transport phenomena, and macroscopic system dynamics, it establishes a cohesive framework for optimizing electrochemical device performance. Key theoretical advances are discussed, including hybrid quantum-mechanics/continuum approaches that capture ionic interactions at atomic resolution, and machine learning-enhanced models that accurately predict microstructural evolution. The review highlights how AI-driven multiscale simulations simultaneously reduce computational demands and enhance predictive power, particularly in assessing material degradation and performance thresholds. Importantly, this work bridges the traditional divide between electrochemical modeling and data science, paving the way for digital twin technologies. By addressing challenges in scale coupling and model validation, this study accelerates the path toward commercial development of high-efficiency hydrogen technologies. These findings are especially relevant for industries pursuing net-zero targets through advanced energy storage solutions, providing both methodological innovations and practical guidance for next-generation fuel cell design.
- Open Access
- Review
Multiscale Simulation in Fuel Cell and Electrolyzer Systems: A Review of Methods, Applications, and Future Prospects
- Kadi Hu 1,
- Bo Li 2, *,
- Ziqi Tian 1, *,
- Liang Chen 1
Author Information
Received: 22 Aug 2025 | Revised: 10 Sep 2025 | Accepted: 28 Sep 2025 | Published: 24 Oct 2025
Abstract
Graphical Abstract
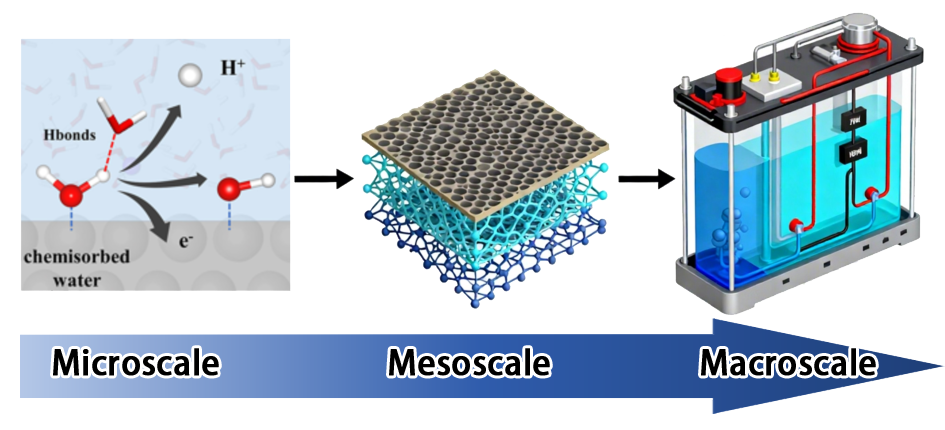
Keywords
fuel cell | electrolyzer | multiscale simulation | machine learning | electrochemical interface
References
- 1.Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; et al. Global hydrogen development—A technological and geopolitical overview. Int. J. Hydrogen Energy 2022, 11, 7016–7048.
- 2.Ferriday, T.B.; Middleton, P.H. Alkaline fuel cell technology—A review. Int. J. Hydrogen Energy 2021, 46, 18489–18510.
- 3.Yang, Y.; Li, P.; Zheng, X.; et al. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 2022, 51, 9620–9693.
- 4.Firouzjaie, H.A.; Mustain, W.E. Catalytic Advantages, Challenges, and Priorities in Alkaline Membrane Fuel Cells. ACS Catal. 2020, 10, 225–234.
- 5.Zhao, G.; Rui, K.; Dou, S.; et al. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291.
- 6.Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180.
- 7.Zhang, X.; Chan, S.H.; Ho, H.K.; et al. Towards a smart energy network: The roles of fuel/electrolysis cells and technological perspectives. Int. J. Hydrogen Energy 2015, 40, 6866–6919.
- 8.Hauch, A.; Kungas, R.; Blennow, P.; et al. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118.
- 9.Zhou, Y.; Zhong, H.; Chen, S.; et al. Proton exchange membrane-based electrocatalytic systems for hydrogen production. Carbon Enery 2024, 7, e629.
- 10.Andreaus, B.; Eikerling, M. Active site model for CO adlayer electrooxidation on nanoparticle catalysts. J. Electroanal. Chem. 2007, 607, 121–132.
- 11.Braatz, R.D.; Alkire, R.C.; Seebauer, E.; et al. Perspectives on the design and control of multiscale systems. J. Process Contr. 2006, 16, 193–204.
- 12.
Blanquer, G.; Yin, Y.; Quiroga, M.A.; et al. Modeling Investigation of the Local Electrochemistry in Lithium-O2 Batteries: A Kinetic Monte Carlo Approach. J. Electrochem. Soc. 2015, 163, A329–A337.
- 13.Shin, H.; Yoo, J.M.; Sung, Y.-E.; et al. Dynamic Electrochemical Interfaces for Energy Conversion and Storage. JACS Au 2022, 2, 2222–2234.
- 14.Magnussen, O.M.; Groß, A. Toward an Atomic-Scale Understanding of Electrochemical Interface Structure and Dynamics. J. Am. Chem. Soc. 2019, 141, 4777–4790.
- 15.Cheng, J.; Liu, X.; VandeVindele, J.; et al. Redox Potentials and Acidity Constants from Density Functional Theory Based Molecular Dynamics. Acc. Chem. Res. 2014, 47, 3522–3529.
- 16.Wang, J.; He, H.; Wu, Y.; et al. Review on Electric Resistance in Proton Exchange Membrane Fuel Cells: Advances and Outlook. Energy Fuels 2024, 38, 2759–2776.
- 17.Zhang, P.; Qiu, H.; Li, H.; et al. Nonmetallic Active Sites on Nickel Phosphide in Oxygen Evolution Reaction. Nanomaterials 2022, 12, 1130.
- 18.Perez Sirkin, Y.A.; Gadea, E.D.; Scherlis, D.A.; et al. Mechanisms of Nucleation and Stationary States of Electrochemically Generated Nanobubbles. J. Am. Chem. Soc. 2019, 141, 10801–10811.
- 19.Li, Q.; Liang, L.; Gerdes, K.; et al. Phase-Field Modeling of Three-Phase Electrode Microstructures in Solid Oxide Fuel Cells. Appl. Phys. Lett. 2012, 101, 033909.
- 20.Picardo, N. 4 Examples of Fuel Cell Modeling in COMSOL Multiphysics. Available online: https://www.comsol.com/blogs/4-examples-of-fuel-cell-modeling-in-comsol-multiphysics (accessed on 16 September 2025).
- 21.Ngo, S.I.; Lim, Y.I. Multiscale Eulerian CFD of Chemical Processes: A Review. ChemEngineering 2020, 4, 23.
- 22.Zaveri, J.; Dhanushkodi, S.R.; Fowler, M.W.; et al. Development of Deep Learning Simulation and Density Functional Theory Framework for Electrocatalyst Layers for PEM Electrolyzers. Energies 2025, 18, 1022.
- 23.Zhao, X.; Liu, Y. Unveiling the Active Structure of Single Nickel Atom Catalysis: Critical Roles of Charge Capacity and Hydrogen Bonding. J. Am. Chem. Soc. 2020, 142, 5773–5777.
- 24.Cohen, A.J.; Mori-Sánchez, P.; Yang, W. Challenges for Density Functional Theory. Chem. Rev. 2012, 112, 289–320.
- 25.Le, J.B.; Chen, A.; Li, L.; et al. Modeling Electrified Pt(111)-Had/Water Interfaces from Ab Initio Molecular Dynamics. JACS Au 2021, 1, 569–577.
- 26.Bursch, M.; Mewes, J.M.; Hansen, A.; et al. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry**. Angew. Chem. Int. Ed. 2022, 61, e202205735.
- 27.
Ju, W.; Bagger, A.; Hao, G.-P.; et al. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944.
- 28.
Yang, H.B.; Hung, S.-F.; Liu, S.; et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147.
- 29.Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81.
- 30.Kim, D.; Shi, J.; Liu, Y. Substantial Impact of Charge on Electrochemical Reactions of Two-Dimensional Materials. J. Am. Chem. Soc. 2018, 140, 9127–9131.
- 31.
Bi, W.; Li, X.; You, R.; et al. Surface Immobilization of Transition Metal Ions on Nitrogen-Doped Graphene Realizing High-Efficient and Selective CO2 Reduction. Adv. Mater. 2018, 30, 1706617.
- 32.Cheng, J.; Sprik, M. Alignment of electronic energy levels at electrochemical interfaces. Phys. Chem. Chem. Phys. 2012, 14, 11245–11267.
- 33.Huang, P.; Pham, T.A.; Galli, G.; et al. Alumina(0001)/Water Interface: Structural Properties and Infrared Spectra from First-Principles Molecular Dynamics Simulations. J. Phys. Chem. C 2014, 118, 8944–8951.
- 34.Le, J.; Iannuzzi, M.; Cuesta, A.; et al. Determining Potentials of Zero Charge of Metal Electrodes versus the Standard Hydrogen Electrode from Density-Functional-Theory-Based Molecular Dynamics. Phys. Rev. Lett. 2017, 119, 016801.
- 35.Cheng, J.; Sprik, M. The electric double layer at a rutile TiO2 water interface modelled using density functional theory based molecular dynamics simulation. J. Phys. Condens. Matter 2014, 26, 244108.
- 36.Surendralal, S.; Todorova, M.; Finnis, M.W.; et al. First-Principles Approach to Model Electrochemical Reactions: Understanding the Fundamental Mechanisms behind Mg Corrosion. Phys. Rev. Lett. 2018, 120, 246801.
- 37.Lan, J.; Rybkin, V.V.; Iannuzzi, M. Ionization of Water as an Effect of Quantum Delocalization at Aqueous Electrode Interfaces. J. Phys. Chem. Lett. 2020, 11, 3724–3730.
- 38.Bouzid, A.; Pasquarello, A. Atomic-Scale Simulation of Electrochemical Processes at Electrode/Water Interfaces under Referenced Bias Potential. J. Phys. Chem. Lett. 2018, 9, 1880–1884.
- 39.Li, X.; Duan, X.; Hua, K.; et al. Local tetragonal distortion of Pt alloy catalysts for enhanced oxygen reduction reaction efficiency. Carbon Enery 2024, 6, e508.
- 40.Yu, C.; Xiang, Y.; Lawson, T.; et al. Graphene oxide-based nanofluidic membranes for reverse electrodialysis that generate electricity from salinity gradients. Carbon Enery 2024, 7, 626.
- 41.Zofchak, E.S.; Zhang, Z.; Marioni, N.; et al. Cation–polymer interactions and local heterogeneity determine the relative order of alkali cation diffusion coefficients in PEGDA hydrogels. J. Membr. Sci. 2023, 685, 121898.
- 42.Borodin, O.; Smith, G.D. Mechanism of Ion Transport in Amorphous Poly(ethylene oxide)/LiTFSI from Molecular Dynamics Simulations. Macromolecules 2006, 39, 1620–1629.
- 43.Marioni, N.; Nordness, O.; Zhang, Z.; et al. Ion and Water Dynamics in the Transition from Dry to Wet Conditions in Salt-Doped PEG. ACS Macro Lett. 2024, 13, 341–347.
- 44.Molinari, N.; Mailoa, J.P.; Kozinsky, B. Effect of Salt Concentration on Ion Clustering and Transport in Polymer Solid Electrolytes: A Molecular Dynamics Study of PEO–LiTFSI. Chem. Mater. 2018, 30, 6298–6306.
- 45.Nordness, O.; Moon, J.D.; Marioni, N.; et al. Probing Water and Ion Diffusion in Functional Hydrogel Membranes by PFG-NMR. Macromolecules 2023, 56, 4669–4680.
- 46.Wheatle, B.K.; Keith, J.R.; Mogurampelly, S.; et al. Influence of Dielectric Constant on Ionic Transport in Polyether-Based Electrolytes. ACS Macro Lett. 2017, 6, 1362–1367.
- 47.Widstrom, M.D.; Borodin, O.; Ludwig, K.B.; et al. Water Domain Enabled Transport in Polymer Electrolytes for Lithium-Ion Batteries. Macromolecules 2021, 54, 2882–2891.
- 48.Feng, G.; Cummings, P.T. Supercapacitor Capacitance Exhibits Oscillatory Behavior as a Function of Nanopore Size. J. Phys. Chem. Lett. 2011, 2, 2859–2864.
- 49.Feng, G.; Li, S.; Atchison, J.S.; et al. Molecular Insights into Carbon Nanotube Supercapacitors: Capacitance Independent of Voltage and Temperature. J. Phys. Chem. C 2013, 117, 9178–9186.
- 50.Hamza, M.; Mei, B.A.; Zeng, Z.; et al. Interfacial thermal signature of electrode/electrolyte interfaces and its effect on charge storage performance during charging of electrochemical energy storage devices. Appl. Therm. Eng. 2025, 274, 126660.
- 51.Scalfi, L.; Salanne, M.; Rotenberg, B. Molecular Simulation of Electrode-Solution Interfaces. Annu. Rev. Phys. Chem. 2021, 72, 189–212.
- 52.Shim, Y.; Kim, H.J.; Jung, Y. Graphene-based supercapacitors in the parallel-plate electrode configuration: Ionic liquidsversus organic electrolytes. Faraday Discuss. 2012, 154, 249–263.
- 53.van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; et al. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409.
- 54.Yang, L.; Fishbine, B.H.; Migliori, A.; et al. Molecular Simulation of Electric Double-Layer Capacitors Based on Carbon Nanotube Forests. J. Am. Chem. Soc. 2009, 131, 12373–12376.
- 55.Yang, L.; Fishbine, B.H.; Migliori, A.; et al. Dielectric saturation of liquid propylene carbonate in electrical energy storage applications. J. Chem. Phys. 2010, 132, 044701.
- 56.Wang, Z.; Yang, Y.; Olmsted, D.L.; et al. Evaluation of the constant potential method in simulating electric double-layer capacitors. J. Chem. Phys. 2014, 141, 184102.
- 57.Holoviak, S.; Dabo, I.; Sinnott, S. Simulation of Electrochemical Oxidation in Aqueous Environments under Applied Voltage Using Classical Molecular Dynamics. J. Phys. Chem. A 2024, 128, 2236–2244.
- 58.Shao, M.; Chang, Q.; Dodelet, J.P.; et al. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657.
- 59.Stevens, M.B.; Anand, M.; Kreider, M.E.; et al. New challenges in oxygen reduction catalysis: A consortium retrospective to inform future research. Energy Environ. Sci. 2022, 15, 3775–3794.
- 60.Thompson, A.P.; Aktulga, H.M.; Berger, R.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171.
- 61.Li, B.; Xiang, W.; Dou, X.; et al. Coarse-Grained Molecular Dynamics Simulation of Nucleation and Stability of Electrochemically Generated Nanobubbles. Langmuir 2025, 41, 8497–8509.
- 62.Ma, Y.; Guo, Z.; Chen, Q.; et al. Dynamic Equilibrium Model for Surface Nanobubbles in Electrochemistry. Langmuir 2021, 37, 2771–2779.
- 63.Li, B.; Ju, M.; Dou, X.; et al. Assessing nanoparticle-surfactant-salt synergistic effects on droplet–droplet electrocoalescence by molecular dynamics simulations. J. Mol. Liq. 2022, 367, 120570.
- 64.Lau, K.C.; Turner, C.; Dunlap, B. Kinetic Monte Carlo simulation of the Yttria Stabilized Zirconia (YSZ) fuel cell cathode. Solid State Ion. 2008, 179, 1912–1920.
- 65.Zhu, H.; Kee, R.J. A general mathematical model for analyzing the performance of fuel-cell membrane-electrode assemblies. J. Power Sources 2003, 117, 61–74.
- 66.Modak, A.; Lusk, M. Kinetic Monte Carlo simulation of a solid-oxide fuel cell: I. Open-circuit voltage and double layer structure. Solid State Ion. 2005, 176, 2181–2191.
- 67.Pornprasertsuk, R.; Cheng, J.; Huang, H.; et al. Electrochemical impedance analysis of solid oxide fuel cell electrolyte using kinetic Monte Carlo technique. Solid State Ion. 2007, 178, 195–205.
- 68.Wei, S.; Luo, Y.; Zhang, H.; et al. Voltage-Dependent Electrochemical Carbon Dioxide Reduction Mechanism Unveiled by Kinetic Monte Carlo Simulation. J. Phys. Chem. Lett. 2025, 16, 2896–2904.
- 69.Wu, L.; Qin, J.; Kharchenko, V.O.; et al. Phase field modeling microstructural evolution of Fe-Cr-Al systems at thermal treatment. Front. Energy Res. 2023, 11, 11:1088742.
- 70.Li, Q.; Xue, D.; Feng, C.; et al. Fracture Simulation of Ni–YSZ Anode Microstructures of Solid Oxide Fuel Cells Using Phase Field Method. J. Electrochem. Soc. 2022, 169, 073507.
- 71.Jiao, Z.; Shikazono, N. Prediction of Nickel Morphological Evolution in Composite Solid Oxide Fuel Cell Anode Using Modified Phase Field Model. J. Electrochem. Soc. 2018, 165, F55.
- 72.Jiao, Z.; Shikazono, N. Simulation of Nickel Morphological and Crystal Structures Evolution in Solid Oxide Fuel Cell Anode Using Phase Field Method. J. Electrochem. Soc. 2014, 161, F577.
- 73.Abdeljawad, F.; Völker, B.; Davis, R.; et al. Connecting microstructural coarsening processes to electrochemical performance in solid oxide fuel cells: An integrated modeling approach. J. Power Sources 2014, 250, 319–331.
- 74.Davis, R.; Abdeljawad, F.; Lillibridge, J.; et al. Phase wettability and microstructural evolution in solid oxide fuel cell anode materials. Acta Mater. 2014, 78, 271–281.
- 75.Hong, L.; Hu, J.M.; Gerdes, K.; et al. Oxygen vacancy diffusion across cathode/electrolyte interface in solid oxide fuel cells: An electrochemical phase-field model. J. Power Sources 2015, 287, 396–400.
- 76.Jiao, Z.; Shikazono, N. Simulation of the reduction process of solid oxide fuel cell composite anode based on phase field method. J. Power Sources 2016, 305, 10–16.
- 77.Kim, J.H.; Liu, W.K.; Lee, C. Multi-scale solid oxide fuel cell materials modeling. Comput. Mech. 2009, 44, 683–703.
- 78.Lei, Y.; Cheng, T.L.; Wen, Y.H. Phase field modeling of microstructure evolution and concomitant effective conductivity change in solid oxide fuel cell electrodes. J. Power Sources 2017, 345, 275–289.
- 79.Liang, L.; Li, Q.; Hu, J.; et al. Phase field modeling of microstructure evolution of electrocatalyst-infiltrated solid oxide fuel cell cathodes. J. Appl. Phys. 2015, 117, 065105.
- 80.Chen, L.Q. Phase-Field Models for Microstructure Evolution. Annu. Rev. Mater. Res. 2002, 32, 113–140.
- 81.Hong, Z.; Viswanathan, V. Open-Sourcing Phase-Field Simulations for Accelerating Energy Materials Design and Optimization. ACS Energy Lett. 2020, 5, 3254–3259.
- 82.Niu, Z.; Pinfield, V.J.; Wu, B.; et al. Towards the digitalisation of porous energy materials: Evolution of digital approaches for microstructural design. Energy Environ. Sci. 2021, 14, 2549–2576.
- 83.Valette, G. Double layer on silver single-crystal electrodes in contact with electrolytes having anions which present a slight specific adsorption: Part I. The (110) face. J. Electroanal. Chem. Interfacial Electrochem. 1981, 122, 285–297.
- 84.Bikerman, J.J. Structure and capacity of electrical double layer. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2009, 33, 384–397.
- 85.
Gu, J.; Liu, S.; Ni, W.; et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 2022, 5, 268–276.
- 86.
Johnson, E.; Haussener, S. Contrasting Views of the Electric Double Layer in Electrochemical CO2 Reduction: Continuum Models vs Molecular Dynamics. J. Phys. Chem. C 2024, 128, 10450–10464.
- 87.Kilic, M.S.; Bazant, M.Z.; Ajdari, A. Steric effects in the dynamics of electrolytes at large applied voltages. II. Modified Poisson-Nernst-Planck equations. Phys. Rev. E 2007, 75, 021503.
- 88.
Ma, Z.; Yang, Z.; Lai, W.; et al. CO2 electroreduction to multicarbon products in strongly acidic electrolyte via synergistically modulating the local microenvironment. Nat. Commun. 2022, 13, 7595.
- 89.
Bohra, D.; Chaudhry, J.H.; Burdyny, T.; et al. Modeling the electrical double layer to understand the reaction environment in a CO2 electrocatalytic system. Energy Environ. Sci. 2019, 12, 3380–3389.
- 90.
Butt, E.N.; Padding, J.T.; Hartkamp, R. Size-modified Poisson–Nernst–Planck approach for modeling a local electrode environment in CO2 electrolysis. Sustain. Energy Fuels 2023, 7, 144–154.
- 91.
Johnson, E.F.; Boutin, E.; Haussener, S. Surface Charge Boundary Condition Often Misused in CO2 Reduction Models. J. Phys. Chem. C 2023, 127, 18784–18790.
- 92.
Johnson, E.F.; Boutin, E.; Liu, S.; et al. Pathways to enhance electrochemical CO2 reduction identified through direct pore-level modeling. EES Catal. 2023, 1, 704–719.
- 93.Wang, H.; Thiele, A.; Pilon, L. Simulations of Cyclic Voltammetry for Electric Double Layers in Asymmetric Electrolytes: A Generalized Modified Poisson–Nernst–Planck Model. J. Phys. Chem. C 2013, 117, 18286–18297.
- 94.
Govindarajan, N.; Lin, T.Y.; Roy, T.; et al. Coupling Microkinetics with Continuum Transport Models to Understand Electrochemical CO2 Reduction in Flow Reactors. PRX Energy 2023, 2, 033010.
- 95.Abdulaziz, M.; Petruk, A.; Samiee, F.; et al. Advancing Hydrodynamic Electrochemistry: A Channel Flow Cell for High-Temperature and High-Pressure Applications. J. Phys. Chem. C 2025, 129, 9726–9735.
- 96.Forschner, L.; Artmann, E.; Jacob, T.; et al. Electric Potential Distribution Inside the Electrolyte during High Voltage Electrolysis. J. Phys. Chem. C 2023, 127, 4387–4394.
- 97.Moorcroft, M.J.; Lawrence, N.S.; Coles, B.A.; et al. High temperature electrochemical studies using a channel flow cell heated by radio frequency radiation. J. Electroanal. Chem. 2001, 506, 28–33.
- 98.Wang, H.; Jusys, Z.; Behm, R.J.; et al. A channel flow cell with double disk electrodes for oxygen electroreduction study at elevated temperatures and pressures: Theory. J. Electroanal. Chem. 2021, 896, 115251.
- 99.Ma, L.; Pourkashanian, M.; Carcadea, E. Review of the Computational Fluid Dynamics Modeling of Fuel Cells. J. Fuel Cell Sci. Technol. 2005, 2, 246–257.
- 100.Akbari, M.H.; Rismanchi, B. Numerical investigation of flow field configuration and contact resistance for PEM fuel cell performance. Renewable Energy 2008, 33, 1775–1783.
- 101.Kloess, J.P.; Wang, X.; Liu, J.; et al. Investigation of bio-inspired flow channel designs for bipolar plates in proton exchange membrane fuel cells. J. Power Sources 2009, 188, 132–140.
- 102.Liu, H.; Li, P.; Lew, J.V. CFD study on flow distribution uniformity in fuel distributors having multiple structural bifurcations of flow channels. Int. J. Hydrogen Energy 2010, 35, 9186–9198.
- 103.Sierra, J.M.; Figueroa-Ramírez, S.J.; Díaz, S.E.; et al. Numerical evaluation of a PEM fuel cell with conventional flow fields adapted to tubular plates. Int. J. Hydrogen Energy 2014, 39, 16694–16705.
- 104.Um, S.; Wang, C.Y. Three-dimensional analysis of transport and electrochemical reactions in polymer electrolyte fuel cells. J. Power Sources 2004, 125, 40–51.
- 105.Vazifeshenas, Y.; Sedighi, K.; Shakeri, M. Numerical investigation of a novel compound flow-field for PEMFC performance improvement. Int. J. Hydrogen Energy 2015, 40, 15032–15039.
- 106.Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; et al. Development of Bi-polar plate design of PEM fuel cell using CFD techniques. Int. J. Hydrogen Energy 2017, 42, 25663–25685.
- 107.Wilberforce, T.; Ijaodola, O.; Emmanuel, O.; et al. Optimization of Fuel Cell Performance Using Computational Fluid Dynamics. Membranes 2021, 11, 146.
- 108.García, M.; Izquierdo, S.; Fueyo, N. Challenges in the electrochemical modelling of solid oxide fuel and electrolyser cells. Renew. Sustain. Energy Rev. 2014, 33, 701–718.
- 109.Hess, B.; Kutzner, C.; van der Spoel, D.; et al. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447.
- 110.Bekas, C.; Curioni, A. Very large scale wavefunction orthogonalization in Density Functional Theory electronic structure calculations. Comput. Phys. Commun. 2010, 181, 1057–1068.
- 111.Fermeglia, M.; Pricl, S. Multiscale molecular modeling in nanostructured material design and process system engineering. Comput. Chem. Eng. 2009, 33, 1701–1710.
- 112.Olsen, J.M.H.; Bolnykh, V.; Meloni, S.; et al. MiMiC: A Novel Framework for Multiscale Modeling in Computational Chemistry. J. Chem. Theory Comput. 2019, 15, 3810–3823.
- 113.Phillips, J.C.; Sun, Y.; Jain, N.; et al. Mapping to Irregular Torus Topologies and Other Techniques for Petascale Biomolecular Simulation. In Proceedings of the International Conference for High Performance Computing, Networking, Storage and Analysis, New Orleans, LA, USA,16–21 November 2014; pp. 81–91.
- 114.Salomon-Ferrer, R.; Götz, A.W.; Poole, D.; et al. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 2. Explicit Solvent Particle Mesh Ewald. J. Chem. Theory Comput. 2013, 9, 3878–3888.
- 115.VandeVondele, J.; Krack, M.; Mohamed, F.; et al. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128.
- 116.AIP Publishing LLC. Multiscale Modeling of Electrochemical Reactions and Processes; AIP Publishing LLC: Melville, NY, USA, 2021. https://doi.org/10.1063/9780735422377.
- 117.Singharoy, A.; Joshi, H.; Ortoleva, P.J. Multiscale Macromolecular Simulation: Role of Evolving Ensembles. J. Chem. Inf. Model. 2012, 52, 2638–2649.
- 118.Delgado-Buscalioni, R.; Coveney, P.V.; Riley, G.D.; et al. Hybrid molecular-continuum fluid models: Implementation within a general coupling framework. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2005, 363, 1975–1985.
- 119.Ingólfsson, H.I.; Bhatia, H.; Aydin, F.; et al. Machine Learning-Driven Multiscale Modeling: Bridging the Scales with a Next-Generation Simulation Infrastructure. J. Chem. Theory Comput. 2023, 19, 2658–2675.
- 120.Vlachos, D.G. Multiscale modeling for emergent behavior, complexity, and combinatorial explosion. AlChE J. 2012, 58, 1314–1325.
- 121.Zhang, Y.; Liu, H.; Yang, W. Free energy calculation on enzyme reactions with an efficient iterative procedure to determine minimum energy paths on a combined ab initio QM/MM potential energy surface. J. Chem. Phys. 2000, 112, 3483–3492.
- 122.Zuckerman, D.M.; Chong, L.T. Weighted Ensemble Simulation: Review of Methodology, Applications, and Software. Annu. Rev. Biophys. 2017, 46, 43–57.
- 123.Bernstein, N.; Várnai, C.; Solt, I.; et al. QM/MM simulation of liquid water with an adaptive quantum region. Phys. Chem. Chem. Phys. 2012, 14, 646–656.
- 124.Dapprich, S.; Komaromi, I.; Byun, K.S.; et al. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. 1999, 461–462, 1–21.
- 125.Heyden, A.; Lin, H.; Truhlar, D.G. Adaptive Partitioning in Combined Quantum Mechanical and Molecular Mechanical Calculations of Potential Energy Functions for Multiscale Simulations. J. Phys. Chem. B 2007, 111, 2231–2241.
- 126.Rowley, C.N.; Roux, B. The Solvation Structure of Na+ and K+ in Liquid Water Determined from High Level ab Initio Molecular Dynamics Simulations. J. Chem. Theory Comput. 2012, 8, 3526–3535.
- 127.Takahashi, H.; Kambe, H.; Morita, A. A simple and effective solution to the constrained QM/MM simulations. J. Chem. Phys. 2018, 148, 134119.
- 128.Takenaka, N.; Kitamura, Y.; Koyano, Y.; et al. The number-adaptive multiscale QM/MM molecular dynamics simulation: Application to liquid water. Chem. Phys. Lett. 2012, 524, 56–61.
- 129.Tzeliou, C.E.; Mermigki, M.A.; Tzeli, D. Review on the QM/MM Methodologies and Their Application to Metalloproteins. Molecules 2022, 27, 2660.
- 130.Vreven, T.; Morokuma, K. Chapter 3 Hybrid Methods: ONIOM(QM:MM) and QM/MM; Elsevier: Amsterdam, The Netherlands, 2006; pp. 35–51.
- 131.Watanabe, H.C.; Kubař, T.; Elstner, M. Size-Consistent Multipartitioning QM/MM: A Stable and Efficient Adaptive QM/MM Method. J. Chem. Theory Comput. 2014, 10, 4242–4252.
- 132.Zhang, R.; Lev, B.; Cuervo, J.E.; et al. A Guide to QM/MM Methodology and Applications. Adv. Quantum Chem. 2010, 59, 353–400.
- 133.Mihai, D.P.; Nitulescu, G.M. Computer-Aided Drug Design and Drug Discovery. Pharmaceuticals 2025, 18, 436.
- 134.Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; et al. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102.
- 135.Bawari, S.; Narayanan, T.N.; Mondal, J. Atomistic Elucidation of Sorption Processes in Hydrogen Evolution Reaction on a van der Waals Heterostructure. J. Phys. Chem. C 2018, 122, 10034–10041.
- 136.Chen, L.; Zhang, X.; Chen, A.; et al. Targeted design of advanced electrocatalysts by machine learning. Chin. J. Catal. 2022, 43, 11–32.
- 137.Du, C.; Qiu, C.; Fang, Z.; et al. Interface hydrophobic tunnel engineering: A general strategy to boost electrochemical conversion of N2 to NH3. Nano Energy 2022, 92, 106784.
- 138.
Hu, X.; Chen, S.; Chen, L.; et al. What is the Real Origin of the Activity of Fe–N–C Electrocatalysts in the O2 Reduction Reaction? Critical Roles of Coordinating Pyrrolic N and Axially Adsorbing Species. J. Am. Chem. Soc. 2022, 144, 18144–18152.
- 139.Wang, X.; Zhu, J.; Kuang, Y.; et al. pH-dependent formation potential of OH* on Pt(111): Double layer effect on water dissociation. Nano Mater. Sci. 2025, 7, 493–499.
- 140.Menezes, P.W.; Indra, A.; Das, C.; et al. Uncovering the Nature of Active Species of Nickel Phosphide Catalysts in High-Performance Electrochemical Overall Water Splitting. ACS Catal. 2016, 7, 103–109.
- 141.Nørskov, J.K.; Bligaard, T.; Hvolbæk, B.; et al. The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 2008, 37, 2163–2171.
- 142.Rossmeisl, J.; Logadottir, A.; Nørskov, J.K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184.
- 143.Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998.
- 144.Stern, L.A.; Feng, L.; Song, F.; et al. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351.
- 145.Verma, A.K.; Verma, A.M.; Govind Rajan, A. Theoretical understanding of electrochemical phenomena in 2D electrode materials. Curr. Opin. Electrochem. 2022, 36, 101116.
- 146.Wang, Y.; Shao, H.; Zhang, C.; et al. Molecular dynamics for electrocatalysis: Mechanism explanation and performance prediction. Energy Rev. 2023, 2, 100028.
- 147.Wang, Y.; Su, H.; He, Y.; et al. Advanced Electrocatalysts with Single-Metal-Atom Active Sites. Chem. Rev. 2020, 120, 12217–12314.
- 148.Wen, S.; Chen, G.; Chen, W.; et al. Nb-doped layered FeNi phosphide nanosheets for highly efficient overall water splitting under high current densities. J. Mater. Chem. A 2021, 9, 9918–9926.
- 149.
Xue, Y.; Guo, Y.; Cui, H.; et al. Catalyst Design for Electrochemical Reduction of CO2 to Multicarbon Products. Small Methods 2021, 5, 2100736.
- 150.Cheng, Q.; Wang, M.; Ni, J.; et al. Comprehensive understanding and rational regulation of microenvironment for gas-involving electrochemical reactions. Carbon Enery 2023, 5, e307.
- 151.Zhang, X.; Wang, J.; Zong, K.; et al. Recent advances in non-noble metal-based electrocatalysts for hybrid water electrolysis systems. Carbon Energy 2025, 7, e679.
- 152.Chen, Q.; Luo, L.; White, H.S. Electrochemical Generation of a Hydrogen Bubble at a Recessed Platinum Nanopore Electrode. Langmuir 2015, 31, 4573–4581.
- 153.German, S.R.; Edwards, M.A.; Chen, Q.; et al. Electrochemistry of single nanobubbles. Estimating the critical size of bubble-forming nuclei for gas-evolving electrode reactions. Faraday Discuss. 2016, 193, 223–240.
- 154.Gupta, S.; Patel, N.; Fernandes, R.; et al. Co–Ni–B nanocatalyst for efficient hydrogen evolution reaction in wide pH range. Appl. Catal. B Environ. 2016, 192, 126–133.
- 155.Luo, L.; White, H.S. Electrogeneration of Single Nanobubbles at Sub-50-nm-Radius Platinum Nanodisk Electrodes. Langmuir 2013, 29, 11169–11175.
- 156.Mao, S.; Wen, Z.; Huang, T.; et al. High-performance bi-functional electrocatalysts of 3D crumpled graphene–cobalt oxide nanohybrids for oxygen reduction and evolution reactions. Energy Environ. Sci. 2014, 7, 609–616.
- 157.Mazloomi, S.K.; Sulaiman, N. Influencing factors of water electrolysis electrical efficiency. Renew. Sustain. Energy Rev. 2012, 16, 4257–4263.
- 158.Zhang, L.; Zhang, Y.; Zhang, X.; et al. Electrochemically Controlled Formation and Growth of Hydrogen Nanobubbles. Langmuir 2006, 22, 8109–8113.
- 159.Zhang, C.; Hu, K.; Liu, X.; et al. Unraveling the Influence of Nafion Content on the Performance of Proton-Exchange Membrane Fuel Cells from the Perspective of Triple-Phase Boundary. Langmuir 2024, 40, 15520–15529.
- 160.
Di Noto, V.; Piga, M.; Giffin, G.A.; et al. Interplay between Mechanical, Electrical, and Thermal Relaxations in Nanocomposite Proton Conducting Membranes Based on Nafion and a [(ZrO2)·(Ta2O5)0.119] Core–Shell Nanofiller. J. Am. Chem. Soc. 2012, 134, 19099–19107.
- 161.Vezzù, K.; Maes, A.M.; Bertasi, F.; et al. Interplay Between Hydroxyl Density and Relaxations in Poly(vinylbenzyltrime thylammonium)-b-poly(methylbutylene) Membranes for Electrochemical Applications. J. Am. Chem. Soc. 2018, 140, 1372–1384.
- 162.Vezzù, K.; Nawn, G.; Negro, E.; et al. Electric Response and Conductivity Mechanism of Blended Polyvinylidene Fluoride/Nafion Electrospun Nanofibers. J. Am. Chem. Soc. 2019, 142, 801–814.
- 163.Wang, Z.; Sun, G.; Lewis, N.H.C.; et al. Water-mediated ion transport in an anion exchange membrane. Nat. Commun. 2025, 16, 1099.
- 164.Gao, M.R.; Zhu, M.N.; Zhang, B.W.; et al. Strategic Design of “Thre-in-One” Cathode Toward Optimal Performance of Proton-Conducting Solid Oxide Fuel Cell: The Temperature Matters. Carbon Enery 2025, 7, e683.
- 165.Wu, Z.; Zhu, P.; Huang, Y.; et al. A Comprehensive Review of Modeling of Solid Oxide Fuel Cells: From Large Systems to Fine Electrodes. Chem. Rev. 2025, 125, 2184–2268.
- 166.Lian, C.; Janssen, M.; Liu, H.; et al. Blessing and Curse: How a Supercapacitor’s Large Capacitance Causes its Slow Charging. Phys. Rev. Lett. 2020, 124, 076001.
- 167.Lin, Y.; Lian, C.; Berrueta, M.U.; et al. Microscopic Model for Cyclic Voltammetry of Porous Electrodes. Phys. Rev. Lett. 2022, 128, 206001.
- 168.Bosio, B.; Bianchi, F.R. Multiscale modelling potentialities for solid oxide fuel cell performance and degradation analysis. Sustain. Energy Fuels 2023, 7, 280–293.
- 169.Bramley, G.A.; Beynon, O.T.; Stishenko, P.V.; et al. The application of QM/MM simulations in heterogeneous catalysis. Phys. Chem. Chem. Phys. 2023, 25, 6562–6585.
- 170.Deng, Z.; Kumar, V.; Bölle, F.T.; et al. Towards autonomous high-throughput multiscale modelling of battery interfaces. Energy Environ. Sci. 2022, 15, 579–594.
- 171.Ding, R.; Chen, J.; Chen, Y.; et al. Unlocking the potential: Machine learning applications in electrocatalyst design for electrochemical hydrogen energy transformation. Chem. Soc. Rev. 2024, 53, 11390–11461.
- 172.Elliott, J.D.; Papaderakis, A.A.; Dryfe, R.A.W.; et al. The electrochemical double layer at the graphene/aqueous electrolyte interface: What we can learn from simulations, experiments, and theory. J. Mater. Chem. C 2022, 10, 15225–15262.
- 173.Kovalenko, A.; Gusarov, S. Multiscale methods framework: Self-consistent coupling of molecular theory of solvation with quantum chemistry, molecular simulations, and dissipative particle dynamics. Phys. Chem. Chem. Phys. 2018, 20, 2947–2969.
- 174.Li, Q.; Ouyang, Y.; Lu, S.; et al. Perspective on theoretical methods and modeling relating to electro-catalysis processes. Chem. Commun. 2020, 56, 9937–9949.
- 175.Senthilnathan, D.; Giunta, P.; Vetere, V.; et al. An efficient and cyclic hydrogen evolution reaction mechanism on [Ni(PH2NH2)2]2+ catalysts: A theoretical and multiscale simulation study. RSC Adv. 2014, 4, 5177–5187.
- 176.Gong, H.; Sun, G.; Shi, W.; et al. Nano-Au-decorated hierarchical porous cobalt sulfide derived from ZIF-67 toward optimized oxygen evolution catalysis: Important roles of microstructures and electronic modulation. Carbon Enery 2024, 6, e432.
- 177.Yuan, Y.; Zheng, Y.; Luo, D.; et al. Recent progress on mechanisms, principles, and strategies for high-activity and high-stability non-PGM fuel cell catalyst design. Carbon Enery 2024, 6, e462.
- 178.Back, S.; Tran, K.; Ulissi, Z.W. Toward a Design of Active Oxygen Evolution Catalysts: Insights from Automated Density Functional Theory Calculations and Machine Learning. ACS Catal. 2019, 9, 7651–7659.
- 179.Mou, T.; Pillai, H.S.; Wang, S.; et al. Bridging the complexity gap in computational heterogeneous catalysis with machine learning. Nat. Catal. 2023, 6, 122–136.
- 180.Fung, V.; Hu, G.; Ganesh, P.; et al. Machine learned features from density of states for accurate adsorption energy prediction. Nat. Commun. 2021, 12, 88.
- 181.
Shang, Z.; Li, H. Distribution of Oxygen Vacancies in RuO2 Catalysts and Their Roles in Activity and Stability in Acidic Oxygen Evolution Reaction. J. Phys. Chem. Lett. 2025, 16, 5418–5428.
- 182.
Shang, Z.; Zhao, S.; Dang, Q.; et al. A General Machine-Learning Framework for High-Throughput Screening for Stable and Efficient RuO2-Based Acidic Oxygen Evolution Reaction Catalysts. ACS Catal. 2025, 15, 12835–12847.
- 183.Takahashi, T.; Ikeda, T.; Murata, K.; et al. Accelerated Durability Testing of Fuel Cell Stacks for Commercial Automotive Applications: A Case Study. J. Electrochem. Soc. 2022, 169, 044523.
- 184.Wang, Y.; Seo, B.; Wang, B.; et al. Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy AI 2020, 1, 100014.
- 185.Yin, P.; Niu, X.; Li, S.-B.; et al. Machine-learning-accelerated design of high-performance platinum intermetallic nanoparticle fuel cell catalysts. Nat. Commun. 2024, 15, 10.
- 186.
Zhong, M.; Tran, K.; Min, Y.; et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 2020, 581, 178–183.
- 187.Konno, N.; Mizuno, S.; Nakaji, H.; et al. Development of Compact and High-Performance Fuel Cell Stack. SAE Int. J. Alt. Power. 2015, 4, 123–129.
- 188.Xu, H.; Cheng, D.; Cao, D.; et al. Revisiting the universal principle for the rational design of single-atom electrocatalysts. Nat. Catal. 2024, 7, 207–218.
- 189.Choubisa, H.; Abed, J.; Mendoza, D.; et al. Accelerated chemical space search using a quantum-inspired cluster expansion approach. Matter 2023, 6, 605–625.
- 190.Jia, Y.; Zhang, R.; Fang, C.; et al. Interpretable Machine Learning To Accelerate the Analysis of Doping Effect on Li/Ni Exchange in Ni-Rich Layered Oxide Cathodes. J. Phys. Chem. Lett. 2024, 15, 1765–1773.
- 191.Ma, N.; Zhang, Y.; Wang, Y.; et al. Machine learning-assisted exploration of the intrinsic factors affecting the catalytic activity of ORR/OER bifunctional catalysts. Appl. Surf. Sci. 2023, 628, 157225.
- 192.Mamun, O.; Winther, K.T.; Boes, J.R.; et al. A Bayesian framework for adsorption energy prediction on bimetallic alloy catalysts. NPJ Comput. Mater. 2020, 6, 177.
- 193.O’Connor, N.J.; Jonayat, A.S.M.; Janik, M.J.; et al. Interaction trends between single metal atoms and oxide supports identified with density functional theory and statistical learning. Nat. Catal. 2018, 1, 531–539.
- 194.Andersen, M.; Levchenko, S.V.; Scheffler, M.; et al. Beyond Scaling Relations for the Description of Catalytic Materials. ACS Catal. 2019, 9, 2752–2759.
- 195.Back, S.; Yoon, J.; Tian, N.; et al. Convolutional Neural Network of Atomic Surface Structures To Predict Binding Energies for High-Throughput Screening of Catalysts. J. Phys. Chem. Lett. 2019, 10, 4401–4408.
- 196.Chen, C.; Ye, W.; Zuo, Y.; et al. Graph Networks as a Universal Machine Learning Framework for Molecules and Crystals. Chem. Mater. 2019, 31, 3564–3572.
- 197.Chen, C.; Zuo, Y.; Ye, W.; et al. A Critical Review of Machine Learning of Energy Materials. Adv. Energy Mater. 2020, 10, 1903242.
- 198.Chen, D.; Shang, C.; Liu, Z.P. Machine-learning atomic simulation for heterogeneous catalysis. NPJ Comput. Mater. 2023, 9, 2.
- 199.Fung, V.; Hu, G.; Sumpter, B. Electronic band contraction induced low temperature methane activation on metal alloys. J. Mater. Chem. A 2020, 8, 6057–6066.
- 200.Goldsmith, B.R.; Esterhuizen, J.; Liu, J.X.; et al. Machine learning for heterogeneous catalyst design and discovery. AlChE J. 2018, 64, 2311–2323.
- 201.Gu, G.H.; Choi, C.; Lee, Y.; et al. Progress in Computational and Machine-Learning Methods for Heterogeneous Small-Molecule Activation. Adv. Mater. 2020, 32, 1907865.
- 202.
Wang, S.; Li, Y.; Dai, S.; et al. Prediction by Convolutional Neural Networks of CO2/N2 Selectivity in Porous Carbons from N2 Adsorption Isotherm at 77 K. Angew. Chem. Int. Ed. 2020, 59, 19645–19648.
- 203.Xie, T.; Grossman, J.C. Crystal Graph Convolutional Neural Networks for an Accurate and Interpretable Prediction of Material Properties. Phys. Rev. Lett. 2018, 120, 145301.
- 204.Wang, Y.; Meyer, Q.; Tang, K.; et al. Large-scale physically accurate modelling of real proton exchange membrane fuel cell with deep learning. Nat. Commun. 2023, 14, 745.
- 205.Niu, Y.; Heydari, A.; Qiu, W.; et al. Machine learning-enabled performance prediction and optimization for iron–chromium redox flow batteries. Nanoscale 2024, 16, 3994–4003.
- 206.Wu, Z.; Chen, F.; Zhou, Z.; et al. Data-driven macro-scale simulation for rapid electrolyte wetting in lithium-ion batteries. J. Energy Storage 2025, 106, 114704.
- 207.Xu, C.; Li, K.; Liu, S.; et al. Identification of Cu-N2 Sites for Zn-Air Batteries in Harsh Electrolytes: Computer Virtual Screening, Machine Learning, and Practical Application. CCS Chem. 2025, 1–15. https://doi.org/10.31635/ccschem.025.202505577
- 208.Aykol, M.; Herring, P.; Anapolsky, A. Machine learning for continuous innovation in battery technologies. Nat. Rev. Mater. 2020, 5, 725–727.
- 209.Kim, S.C.; Oyakhire, S.T.; Athanitis, C.; et al. Data-driven electrolyte design for lithium metal anodes. Proc. Natl. Acad. Sci. USA 2023, 120, e2214357120.
- 210.Makki Abadi, M.; Rashidi, M.M. Machine Learning for the Optimization and Performance Prediction of Solid Oxide Electrolysis Cells: A Review. Processes 2025, 13, 875.
- 211.Aldoseri, A.; Al-Khalifa, K.N.; Hamouda, A.M. Re-Thinking Data Strategy and Integration for Artificial Intelligence: Concepts, Opportunities, and Challenges. Appl. Sci. 2023, 13, 7082.
- 212.Sendek, A.D.; Cubuk, E.D.; Antoniuk, E.R.; et al. Machine Learning-Assisted Discovery of Solid Li-Ion Conducting Materials. Chem. Mater. 2018, 31, 342–352.
- 213.Yu, H.; Zhang, L.; Wang, W.; et al. Lithium-ion battery multi-scale modeling coupled with simplified electrochemical model and kinetic Monte Carlo model. iScience 2023, 26, 107661.
- 214.Tao, H.; Wang, S.; Liu, H.; et al. Deep Neural Network Enhanced Mesoscopic Thermodynamic Model for Unlocking the Electrode/Electrolyte Interface. Angew. Chem. Int. Ed. 2025, 64, e202418447.
- 215.Huang, P.; Leng, Y.; Lian, C.; et al. Porous-DeepONet: Learning the Solution Operators of Parametric Reactive Transport Equations in Porous Media. Engineering 2024, 39, 101–111.
- 216.Zhu, Q.; Huang, Y.; Zhou, D.; et al. Automated synthesis of oxygen-producing catalysts from Martian meteorites by a robotic AI chemist. Nat. Synth. 2024, 3, 319–328.
- 217.Fu, R.; Wang, L.; Yang, X.; et al. Defects-Engineered Metal-Organic Frameworksfor Supercapacitor Platform. Sustain. Eng. Novit 2025, 1, 2.
- 218.Song, X.; Ge, Y,; Xue, X.; et al. Electrolyte-triggered in-situ polymerization of multi-site organic cathodes for superior-longevity cation-anion co-storage secondary batteries. Chem. Eng. J. 2024, 499, 156359.
- 219.Zhou, X.; Wang, Y.; Gu, Y.; et al. All-Purpose Redox-Active Metal–Organic Frameworks as Both Cathodic and Anodic Host Materials for Advanced Lithium-Sulfur Batteries. Matter 2024, 7, 3069–3082.
- 220.Liu, S.; Tian, B.; Wang, X.; et al. The Critical Role of Initial/Operando Oxygen Loading in General Bismuth-Based Catalysts for Electroreduction of Carbon Dioxide. J. Phys. Chem. Lett. 2022, 13, 9607–9617.

This work is licensed under a Creative Commons Attribution 4.0 International License.


