This paper explores the challenges that arise when performing and interpreting leaf gas exchange measurements in plants subjected to abiotic stress. It highlights how factors such as cuticular fluxes, stomatal closure, and common assumptions about gas exchange can lead to errors, especially under stress conditions. Key phenomena such as substomatal cavity unsaturation and stomatal patchiness during water stress are discussed in detail, as they significantly complicate the calculation of gas exchange parameters under stress. The paper also addresses the importance of other factors, including steady-state conditions, the differences between adaxial and abaxial surface responses, and boundary layer effects, all of which play critical roles in influencing the accuracy of measurements. Important physiological indicators—such as intrinsic water-use efficiency, minimum leaf conductance, substomatal CO2 concentration, and mesophyll conductance—are analysed in the context of how stress-induced discrepancies in data often result from measurement artefacts rather than true physiological differences. To address these challenges, the paper outlines practical approaches to improving measurement accuracy, offering insights on standardising experimental conditions and minimising errors. By recognising these issues, gaps in current knowledge are identified, providing a comprehensive overview of the challenges in interpreting leaf gas exchange data under stress conditions and suggesting areas for further study.
- Open Access
- Review
Navigating Challenges in Interpreting Plant Physiology Responses through Gas Exchange Results in Stressed Plants
Author Information
Received: 14 Nov 2024 | Revised: 20 Dec 2024 | Accepted: 27 Dec 2024 | Published: 13 Jan 2025
Abstract
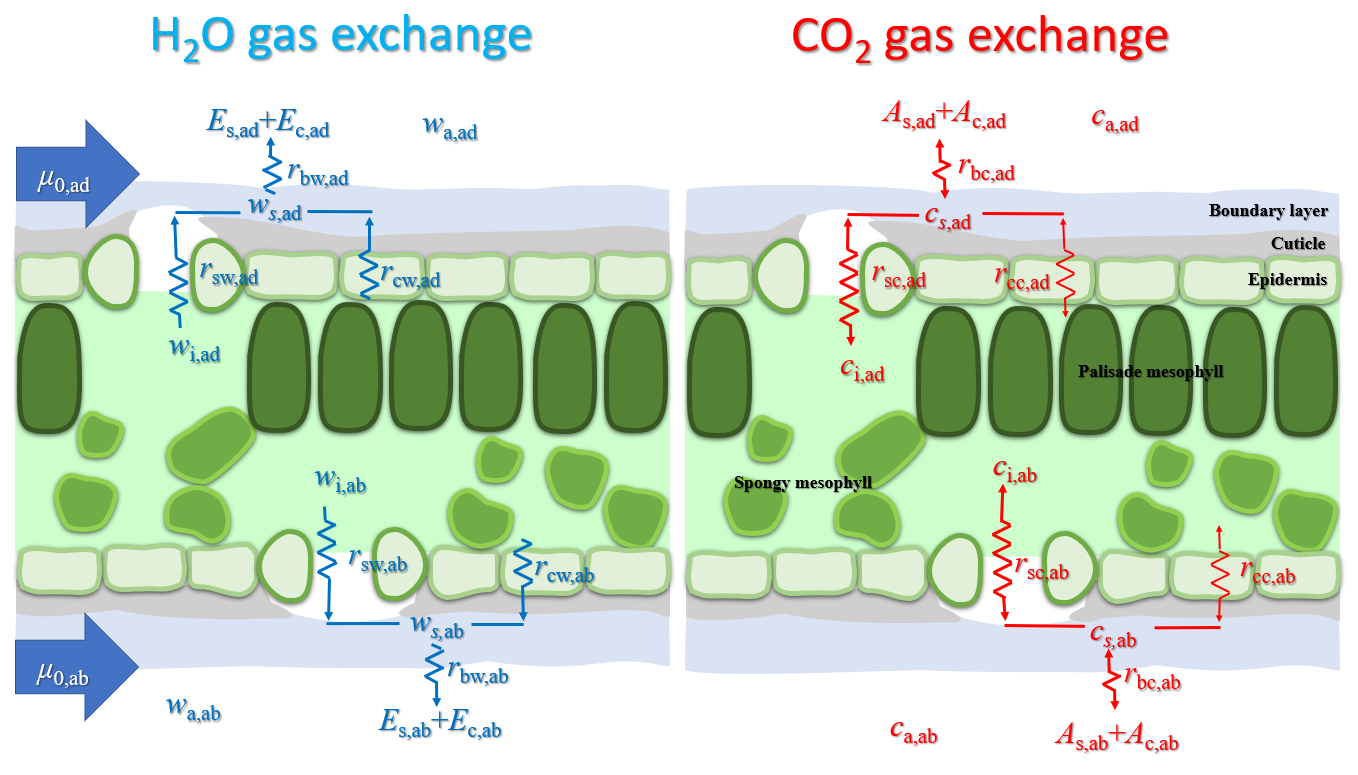
Graphical Abstract
Keywords
plant stress | cuticular conductance | stomatal patchiness | unsaturation | abaxial | adaxial | leaf gas exchange
References
- 1.Boyer JS. (2015a). Impact of cuticle on calculations of the CO2 concentration inside leaves. Planta, 242, 1405–1412.
- 2.Boyer JS. (2015b). Turgor and the transport of CO2 and water across the cuticle (epidermis) of leaves. Journal of Experimental Botany, 66(9), 2625–2633.
- 3.Boyer JS, Wong SC, & Farquhar GD. (1997). CO2 and water vapor exchange across leaf cuticle (Epidermis) at various water potentials. Plant Physiology, 114, 185–191.
- 4.Buckley TN, & Sack L. (2019). The humidity inside leaves and why you should care: implications of unsaturation of leaf intercellular airspaces. American Journal of Botany, 106(5), 618–621. https://doi.org/10.1002/ajb2.1282
- 5.Burghardt M, & Riederer M. (2006). Cuticular Transpiration. In M Riederer & C Muller (Eds.), Annual Plant Reviews Volume 23: Biology of the Plant Cuticle (Vol. 23, pp. 292–311). https://doi.org/10.1002/9780470988718.ch9
- 6.Busch FA, Ainsworth EA, Amtmann A, Cavanagh AP, Driever SM, Ferguson JN, Kromdijk J, Lawson T, Leakey ADB, Matthews JSA, Meacham-Hensold K, Vath RL, Vialet-Chabrand S, Walker BJ, & Papanatsiou M. (2024). A guide to photosynthetic gas exchange measurements: Fundamental principles, best practice and potential pitfalls. Plant, Cell & Environment, 47(9), 3344–3364. https://doi.org/10.1111/pce.14815
- 7.Busch FA, Holloway-Phillips M, Stuart-Williams H, & Farquhar GD. (2020). Revisiting carbon isotope discrimination in C3 plants shows respiration rules when photosynthesis is low. Nature Plants, 6(3), 245–258. https://doi.org/10.1038/s41477-020-0606-6
- 8.Busch FA, & Sage RF. (2017). The sensitivity of photosynthesis to O2 and CO2 concentration identifies strong Rubisco control above the thermal optimum. New Phytologist, 213(3), 1036–1051. https://doi.org/10.1111/nph.14258
- 9.Busch FA, Sage TL, Cousins AB, & Sage RF. (2013). C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant, Cell & Environment, 36(1), 200–212. https://doi.org/10.1111/j.1365-3040.2012.02567.x
- 10.Caird MA, Richards JH, & Donovan LA. (2007). Nighttime Stomatal Conductance and Transpiration in C3 and C4 Plants. Plant Physiology, 143(1), 4–10. https://doi.org/10.1104/pp.106.092940
- 11.Cardon ZG, Mott KA, & Berry JA. (1994). Dynamics of patchy stomatal movements, and their contribution to steady-state and oscillating stomatal conductance calculated using gas-exchange techniques. Plant, Cell & Environment, 17(9), 995–1007. https://doi.org/10.1111/j.1365-3040.1994.tb02033.x
- 12.Cernusak LA, Goldsmith GR, Arend M, & Siegwolf RTW. (2019). Effect of vapor pressure deficit on gas exchange in wild-type and abscisic acid–insensitive plants. Plant Physiology, 181(4), 1573–1586. https://doi.org/10.1104/pp.19.00436
- 13.Cernusak LA, Ubierna N, Jenkins MW, Garrity SR, Rahn T, Powers HH, Hanson DT, Sevanto S, Wong SC, McDowell NG, & Farquhar GD. (2018). Unsaturation of vapour pressure inside leaves of two conifer species. Scientific Reports, 8(7667), 1–7.
- 14.Cernusak LA, Wong SC, Stuart-Williams H, Márquez DA, Pontarin N, & Farquhar GD. (2024). Unsaturation in the air spaces of leaves and its implications. Plant, Cell & Environment, 47(10), 3685–3698. https://doi.org/10.1111/pce.15001
- 15.Clemente-Moreno MJ, Gago J, Díaz-Vivancos P, Bernal A, Miedes E, Bresta P, Liakopoulos G, Fernie AR, Hernández JA, & Flexas J. (2019). The apoplastic antioxidant system and altered cell wall dynamics influence mesophyll conductance and the rate of photosynthesis. Plant Journal, 99(6), 1031–1046. https://doi.org/10.1111/tpj.14437
- 16.Collaviti SE, Stuart-Williams H, Farquhar GD, Cernusak LA, & Márquez DA. (2024). Unsaturation and approximate isotopic homogeneity in leaf air spaces. bioRxiv. https://doi.org/10.1101/2024.09.30.610858
- 17.Condon AG, Richards RA, Rebetzke GJ, & Farquhar GD. (2004). Breeding for high water-use efficiency. Journal of Experimental Botany, 55(407), 2447–2460. https://doi.org/10.1093/jxb/erh277
- 18.Coupel-Ledru A, Lebon E, Christophe A, Gallo A, Gago P, Pantin F, Doligez A, & Simonneau T. (2016). Reduced nighttime transpiration is a relevant breeding target for high water-use efficiency in grapevine. Proceedings of the National Academy of Sciences, 113(32), 8963–8968. https://doi.org/10.1073/pnas.1600826113
- 19.Cowan IR. (1972). Mass and heat transfer in laminar boundary layers with particular reference to assimilation and transpiration in leaves. Agricultural Meteorology, 10, 311–329.
- 20.Cowan IR, & Farquhar GD. (1977). Stomatal function in relation to leaf metabolism and environment. Symp Soc Exp Biol, 31, 471–505.
- 21.Deans RM, Brodribb TJ, Busch FA, & Farquhar GD. (2020). Optimization can provide the fundamental link between leaf photosynthesis, gas exchange and water relations. Nature Plants, 6(9), 1116–1125. https://doi.org/10.1038/s41477-020-00760-6
- 22.Downton WJS, Loveys BR, & Grant WJR. (1988). Non-Uniform Stomatal Closure Induced by Water Stress Causes Putative Non-Stomatal Inhibition of Photosynthesis. The New Phytologist, 110(4), 503–509. http://www.jstor.org/stable/2434912
- 23.Duursma RA, Blackman CJ, Lopez R, Martin-StPaul NK, Cochard H, & Medlyn BE. (2018). On the minimum leaf conductance: its role in models of plant water use, and ecological and environmental control. New Phytologist, (221), 693–705. https://doi.org/10.1111/nph.15395
- 24.Evans JR, Sharkey TD, Berry JA, & Farquhar GD. (1986). Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Functional Plant Biology, 13(2), 281–292. https://doi.org/10.1071/PP9860281
- 25.Farquhar GD, Griffani DS, & Barbour MM. (2021). The effects on isotopic composition of leaf water and transpiration of adding a gas-exchange cuvette. Plant, Cell & Environment, 44(9), 2844–2857. https://doi.org/10.1111/pce.14076
- 26.Farquhar GD, & Raschke K. (1978). On the resistance to transpiration of the sites of evaporation within the leaf. Plant Physiology, 61(6), 1000–1005. https://doi.org/10.1104/pp.61.6.1000
- 27.Farquhar GD, & Richards RA. (1984). Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes [Article]. Australian Journal of Plant Physiology, 11(6), 539–552. https://doi.org/10.1071/pp9840539
- 28.Farquhar GD, & Sharkey TD. (1982). Stomatal conductance and photosynthesis. Annual Review Plant Physiology, 33, 317–345. https://doi.org/10.1146/annurev.pp.33.060182.001533
- 29.Farquhar GD, von Caemmerer S, & Berry JA. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149(1), 78–90. https://doi.org/10.1007/BF00386231
- 30.Flexas J, Díaz-Espejo A, Berry J, Cifre J, Galmés J, Kaldenhoff R, Medrano H, & Ribas-Carbó M. (2007a). Analysis of leakage in IRGA's leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. Journal of Experimental Botany, 58(6), 1533–1543. https://doi.org/10.1093/jxb/erm027
- 31.Flexas J, Diaz-Espejo A, Galmes J, Kaldenhoff R, Medrano H, & Ribas-Carbo M. (2007b). Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell & Environment, 30(10), 1284–1298. https://doi.org/10.1111/j.1365-3040.2007.01700.x
- 32.Flexas J, Niinemets Ü, Gallé A, Barbour MM, Centritto M, Diaz-Espejo A, Douthe C, Galmés J, Ribas-Carbo M, Rodriguez PL, Rosselló F, Soolanayakanahally R, Tomas M, Wright IJ, ... Medrano H. (2013). Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Research, 117(1), 45–59. https://doi.org/10.1007/s11120-013-9844-z
- 33.Fu Z, Ciais P, Wigneron J-P, Gentine P, Feldman AF, Makowski D, Viovy N, Kemanian AR, Goll DS, Stoy PC, Prentice IC, Yakir D, Liu L, Ma H,…Smith WK. (2024). Global critical soil moisture thresholds of plant water stress. Nature Communications, 15(1), 4826. https://doi.org/10.1038/s41467-024-49244-7
- 34.Gaastra P. (1959). Photosynthesis of crop plants as influenced by light, carbon dioxide, temperature, and stomatal diffusion resistance. Meded. Landbouwhogeschool, Wageningen.
- 35.Garen JC, Branch HA, Borrego I, Blonder B, Stinziano JR, & Michaletz ST. (2022). Gas exchange analysers exhibit large measurement error driven by internal thermal gradients. New Phytologist, 236(2), 369–384. https://doi.org/10.1111/nph.18347
- 36.Grantz DA, Karr M, & Burkhardt J. (2020). Heterogeneity of Stomatal Pore Area Is Suppressed by Ambient Aerosol in the Homobaric Species, Vicia faba. Front Plant Sci, 11, 897. https://doi.org/10.3389/fpls.2020.00897
- 37.Grassi G, & Magnani F. (2005). Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell & Environment, 28(7), 834–849. https://doi.org/10.1111/j.1365-3040.2005.01333.x
- 38.Griffani DS, Rognon P, & Farquhar GD. (2024). The role of thermodiffusion in transpiration. New Phytologist, 243(4), 1301–1311. https://doi.org/10.1111/nph.19642
- 39.Grossiord C, Buckley TN, Cernusak LA, Novick KA, Poulter B, Siegwolf RTW, Sperry JS, & McDowell NG. (2020). Plant responses to rising vapor pressure deficit. New Phytologist, 226(6), 1550–1566. https://doi.org/10.1111/nph.16485
- 40.Hanson D, Stutz SS, & Boyer JS. (2016). Why small fluxes matter: the case and approaches for improving measurements of photosynthesis and (photo) respiration. Journal of Experimental Botany, 67, 3027–3039.
- 41.Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, & Evans JR. (2009). Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. Journal of Experimental Botany, 60(8), 2303–2314. https://doi.org/10.1093/jxb/erp021
- 42.Holmgren P, Jarvis P, & Jarvis M. (1965). Resistances to carbon dioxide and water vapour transfer in leaves of different plant species. Physiologia Plantarum, 18, 557–573.
- 43.Hussain SB, Stinziano J, Pierre MO, & Vincent C. (2024). Accurate photosynthetic parameter estimation at low stomatal conductance: effects of cuticular conductance and instrumental noise. Photosynthesis Research, 160(2), 111–124. https://doi.org/10.1007/s11120-024-01092-8
- 44.Jain P, Huber AE, Rockwell FE, Sen S, Holbrook NM, & Stroock AD. (2023). Localized measurements of water potential reveal large loss of conductance in living tissues of maize leaves. Plant Physiology, 194(4), 2288–2300. https://doi.org/10.1093/plphys/kiad679
- 45.Jain P, Huber AE, Rockwell FE, Sen S, Holbrook NM, & Stroock AD. (2024). New approaches to dissect leaf hydraulics reveal large gradients in living tissues of tomato leaves. New Phytologist, 242(2), 453–465. https://doi.org/10.1111/nph.19585
- 46.Jain P, Liu W, Zhu S, Chang CY-Y, Melkonian J, Rockwell FE, Pauli D, Sun Y, Zipfel WR, Holbrook NM, Riha SJ, Gore MA, & Stroock AD. (2021). A minimally disruptive method for measuring water potential in planta using hydrogel nanoreporters. Proceedings of the National Academy of Sciences, 118(23), e2008276118. https://doi.org/10.1073/pnas.2008276118
- 47.Jarvis PG, & Slatyer RO. (1970). The role of the mesophyll cell wall in leaf transpiration. Planta, 90(4), 303–322. https://doi.org/10.1007/BF00386383
- 48.Kaiser H, & Kappen L. (1997). In situ observations of stomatal movements in different light-dark regimes: the influence of endogenous rhythmicity and long-term adjustments1. Journal of Experimental Botany, 48(8), 1583–1589. https://doi.org/10.1093/jxb/48.8.1583
- 49.Kaiser H, & Kappen L. (2000). In situ observation of stomatal movements and gas exchange of Aegopodium podagraria L. in the understorey. Journal of Experimental Botany, 51(351), 1741–1749. https://doi.org/10.1093/jexbot/51.351.1741
- 50.Kerstiens G. (1996). Cuticular water permeability and its physiological significance. Journal of Experimental Botany, 47(305), 1813–1832.
- 51.Kitao M, Harayama H, & Uemura A. (2017). A practical approach to estimate diffusional leakages of leaf chamber of open gas exchange systems using intact leaves. Plant, Cell & Environment, 40(11), 2870–2874. https://doi.org/10.1111/pce.13032
- 52.Laisk A. (1983). Calculation of Leaf Photosynthetic Parameters Considering the Statistical Distribution of Stomatal Apertures. Journal of Experimental Botany, 34(12), 1627–1635. https://doi.org/10.1093/jxb/34.12.1627
- 53.Lawson T, Weyers J, & A'Brook R. (1998). The nature of heterogeneity in the stomatal behaviour of Phaseolus vulgaris L. primary leaves. Journal of Experimental Botany, 49(325), 1387–1395. https://doi.org/10.1093/jxb/49.325.1387
- 54.Leakey ADB, Ferguson JN, Pignon CP, Wu A, Jin Z, Hammer GL, & Lobell DB. (2019). Water Use Efficiency as a Constraint and Target for Improving the Resilience and Productivity of C3 and C4 Crops. Annual Review of Plant Biology, 70(Volume 70, 2019), 781–808. https://doi.org/10.1146/annurev-arplant-042817-040305
- 55.Liang J, Krauss KW, Finnigan J, Stuart-Williams H, Farquhar GD, & Ball MC. (2023). Linking water use efficiency with water use strategy from leaves to communities. New Phytologist, 240(5), 1735–1742. https://doi.org/10.1111/nph.19308
- 56.LICOR B. (2020). Using the LI-6800 Version 1.4. LI-COR, Inc. https://licor.app.boxenterprise.net/s/2yrup4d2nq0n07sur6a7xph893tyr284
- 57.Long SP, & Bernacchi CJ. (2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany, 54(392), 2393–2401. https://doi.org/10.1093/jxb/erg262
- 58.Márquez DA, & Busch FA. (2024). The interplay of short-term mesophyll and stomatal conductance responses under variable environmental conditions. Plant, Cell & Environment, 47(9), 3393–3410. https://doi.org/10.1111/pce.14880
- 59.Márquez DA, Stuart-Williams H, Cernusak LA, & Farquhar GD. (2023a). Assessing the CO2 concentration at the surface of photosynthetic mesophyll cells. New Phytologist, 238(4), 1446–1460. https://doi.org/10.1111/nph.18784
- 60.Márquez DA, Stuart-Williams H, & Farquhar GD. (2021). An improved theory for calculating leaf gas exchange more precisely accounting for small fluxes. Nature Plants, 7, 317–326. https://doi.org/10.1038/s41477-021-00861-w
- 61.Márquez DA, Stuart-Williams H, Farquhar GD, & Busch FA. (2021). Cuticular conductance of adaxial and abaxial leaf surfaces and its relation to minimum leaf surface conductance. New Phytologist, 233(1), 156–168. https://doi.org/10.1111/nph.17588
- 62.Márquez DA, Stuart-Williams H, Wong SC, & Farquhar GD. (2023b). An improved system to measure leaf gas exchange on adaxial and abaxial surfaces. Bio-protocol, 13(11), e4687. https://doi.org/10.21769/BioProtoc.4687
- 63.Márquez DA, Wong SC, Stuart-Williams H, Cernusak LA, & Farquhar GD. (2024). Mesophyll airspace unsaturation drives C4 plant success under vapour pressure deficit stress. Proceedings of the National Academy of Sciences, 121(39). https://doi.org/10.1073/pnas.2402233121
- 64.Massman WJ. (1998). A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO, and NO2 in air, O2 and N2 near STP. Atmospheric Environment, 32(6), 1111–1127. https://doi.org/10.1016/S1352-2310(97)00391-9
- 65.Moss DN, & Rawlins SL. (1963). Concentration of carbon dioxide inside leaves. Nature, 197(4874), 1320–1321. https://doi.org/10.1038/1971320a0
- 66.Mott KA. (1995). Effects of patchy stomatal closure on gas exchange measurements following abscisic acid treatment. Plant, Cell & Environment, 18(11), 1291–1300. https://doi.org/10.1111/j.1365-3040.1995.tb00188.x
- 67.Mott KA, & Buckley TN. (2000). Patchy stomatal conductance: emergent collective behaviour of stomata. Trends in Plant Science, 5(6), 258–262. https://doi.org/10.1016/S1360-1385(00)01648-4
- 68.Mott KA, Cardon ZG, & Berry JA. (1993). Asymmetric patchy stomatal closure for the two surfaces of Xanthium strumarium L. leaves at low humidity. Plant, Cell & Environment, 16(1), 25–34. https://doi.org/10.1111/j.1365-3040.1993.tb00841.x
- 69.Parkhurst DF, Wong SC, Farquhar GD, & Cowan IR. (1988). Gradients of intercellular CO2 levels across the leaf mesophyll. Plant Physiology, 86(4), 1032–1037. https://doi.org/10.1104/pp.86.4.1032
- 70.Resco de Dios V, Chowdhury FI, Granda E, Yao Y, & Tissue DT. (2019). Assessing the potential functions of nocturnal stomatal conductance in C3 and C4 plants. New Phytologist, 223(4), 1696–1706. https://doi.org/10.1111/nph.15881
- 71.Rockwell FE, Holbrook NM, Jain P, Huber AE, Sen S, & Stroock AD. (2022). Extreme undersaturation in the intercellular airspace of leaves: a failure of Gaastra or Ohm? Annals of Botany, 130(3), 301–316. https://doi.org/10.1093/aob/mcac094
- 72.Roig-Oliver M, Nadal M, Clemente-Moreno MJ, Bota J, & Flexas J. (2020). Cell wall components regulate photosynthesis and leaf water relations of Vitis vinifera cv. Grenache acclimated to contrasting environmental conditions. Journal of Plant Physiology, 244, 153084. https://doi.org/10.1016/j.jplph.2019.153084
- 73.Scholander PF, Bradstreet ED, Hemmingsen EA, & Hammel HT. (1965). Sap Pressure in Vascular Plants. Science, 148(3668), 339–346. https://doi.org/10.1126/science.148.3668.339
- 74.Schreiber L. (2001). Effect of temperature on cuticular transpiration of isolated cuticular membranes and leaf discs. Journal of Experimental Botany, 52(362), 1893–1900. https://doi.org/10.1093/jexbot/52.362.1893
- 75.Schuepp PH. (1993). Tansley Review No. 59. Leaf Boundary Layers. The New Phytologist, 125(3), 477-507. http://www.jstor.org/stable/2558258
- 76.Sharkey TD. (2016). What gas exchange data can tell us about photosynthesis. Plant, Cell & Environment, 39(6), 1161–1163. https://doi.org/10.1111/pce.12641
- 77.Slot M, Nardwattanawong T, Hernández GG, Bueno A, Riederer M, & Winter K. (2021). Large differences in leaf cuticle conductance and its temperature response among 24 tropical tree species from across a rainfall gradient. New Phytologist, 232(4), 1618–1631. https://doi.org/10.1111/nph.17626
- 78.Sun Z, Song Y, Li Q, Cai J, Wang X, Zhou Q, Huang M, & Jiang D. (2021). An Integrated Method for Tracking and Monitoring Stomata Dynamics from Microscope Videos. Plant Phenomics, 2021. https://doi.org/10.34133/2021/9835961
- 79.Tazoe Y, von Caemmerer S, Estavillo GM, & Evans JR. (2011). Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant, Cell & Environment, 34(4), 580–591. https://doi.org/10.1111/j.1365-3040.2010.02264.x
- 80.Tholen D. (2024). GasanalyzeR: advancing reproducible research using a new R package for photosynthesis data workflows. AoB PLANTS, 16(4). https://doi.org/10.1093/aobpla/plae035
- 81.Tominaga J, & Kawamitsu Y. (2015). Cuticle affects calculations of internal CO2 in leaves closing their stomata. Plant and Cell Physiology, 56(10), 1900–1908.
- 82.Turner NC, Schulze ED, & Gollan T. (1984). The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content. Oecologia, 63(3), 338–342. https://doi.org/10.1007/BF00390662
- 83.von Caemmerer S. (2000). Biochemical models of leaf photosynthesis. CSIRO Publishing. https://doi.org/10.1071/9780643103405
- 84.von Caemmerer S. (2013). Steady-state models of photosynthesis. Plant, Cell & Environment, 36(9), 1617–1630. https://doi.org/10.1111/pce.12098
- 85.von Caemmerer S, & Farquhar GD. (1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta, 153, 376–387.
- 86.Vrábl D, Vašková M, Hronková M, Flexas J, & Šantrůček J. (2009). Mesophyll conductance to CO2 transport estimated by two independent methods: effect of variable CO2 concentration and abscisic acid. Journal of Experimental Botany, 60(8), 2315–2323. https://doi.org/10.1093/jxb/erp115
- 87.Wall S, Vialet-Chabrand S, Davey P, Van Rie J, Galle A, Cockram J, & Lawson T. (2022). Stomata on the abaxial and adaxial leaf surfaces contribute differently to leaf gas exchange and photosynthesis in wheat. New Phytologist, 235(5), 1743–1756. https://doi.org/10.1111/nph.18257
- 88.Wang S, Hoch G, Grun G, & Kahmen A. (2024). Water loss after stomatal closure: quantifying leaf minimum conductance and minimal water use in nine temperate European tree species during a severe drought. Tree Physiology, 44(4). https://doi.org/10.1093/treephys/tpae027
- 89.Wong SC, Canny MJ, Holloway-Phillips M, Stuart-Williams H, Cernusak LA, Márquez DA, & Farquhar GD. (2022). Humidity gradients in the air spaces of leaves. Nature Plants, 8, 971–978. https://doi.org/10.1038/s41477-022-01202-1
- 90.Wong SC, Cowan IR, & Farquhar GD. (1979). Stomatal conductance correlates with photosynthetic capacity. Nature, 282(5737), 424–426. https://doi.org/10.1038/282424a0
- 91.Wong SC, Cowan IR, & Farquhar GD. (1985a). Leaf conductance in relation to rate of CO2 assimilation I. Influence of nitrogen nutrition, phosphorus nutrition, photon flux density, and ambient partial pressure of CO2 during ontogeny. Plant Physiology, 78(4), 821–825. https://doi.org/10.1104/pp.78.4.821
- 92.Wong SC, Cowan IR, & Farquhar GD. (1985b). Leaf conductance in relation to rate of CO2 assimilation II. Effects of short-term exposures to different photon flux densities assimilation. Plant Physiology, 78(4), 826–829. https://doi.org/10.1104/pp.78.4.826
- 93.Wong SC, Cowan IR, & Farquhar GD. (1985c). Leaf conductance in relation to rate of CO2 assimilation III. Influences of water stress and photoinhibition. Plant Physiology, 78(4), 830–834. https://doi.org/10.1104/pp.78.4.830
- 94.Xiong D, Liu XI, Liu L, Douthe C, Li Y, Peng S, & Huang J. (2015). Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant, Cell & Environment, 38, 2541–2550. https://doi.org/10.1111/pce.12558
- 95.Yan W, Zhong Y, & Shangguan Z. (2016). A meta-analysis of leaf gas exchange and water status responses to drought. Scientific Reports, 6(1), 20917. https://doi.org/10.1038/srep20917
- 96.Yu K, Goldsmith GR, Wang Y, & Anderegg WRL. (2019). Phylogenetic and biogeographic controls of plant nighttime stomatal conductance. New Phytologist, 222(4), 1778–1788. https://doi.org/10.1111/nph.15755
- 97.Yu YZ, Ma WT, Wang X, Tcherkez G, Schnyder H, & Gong XY. (2024). Reconciling water-use efficiency estimates from carbon isotope discrimination of leaf biomass and tree rings: Nonphotosynthetic fractionation matters. New Phytologist, 244(6). 2225–2238. https://doi.org/10.1111/nph.20170
How to Cite
Márquez, D. A., Gardner, A., & Busch, F. A. (2025). Navigating Challenges in Interpreting Plant Physiology Responses through Gas Exchange Results in Stressed Plants. Plant Ecophysiology, 1(1), 2. https://doi.org/10.53941/plantecophys.2025.100002
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


