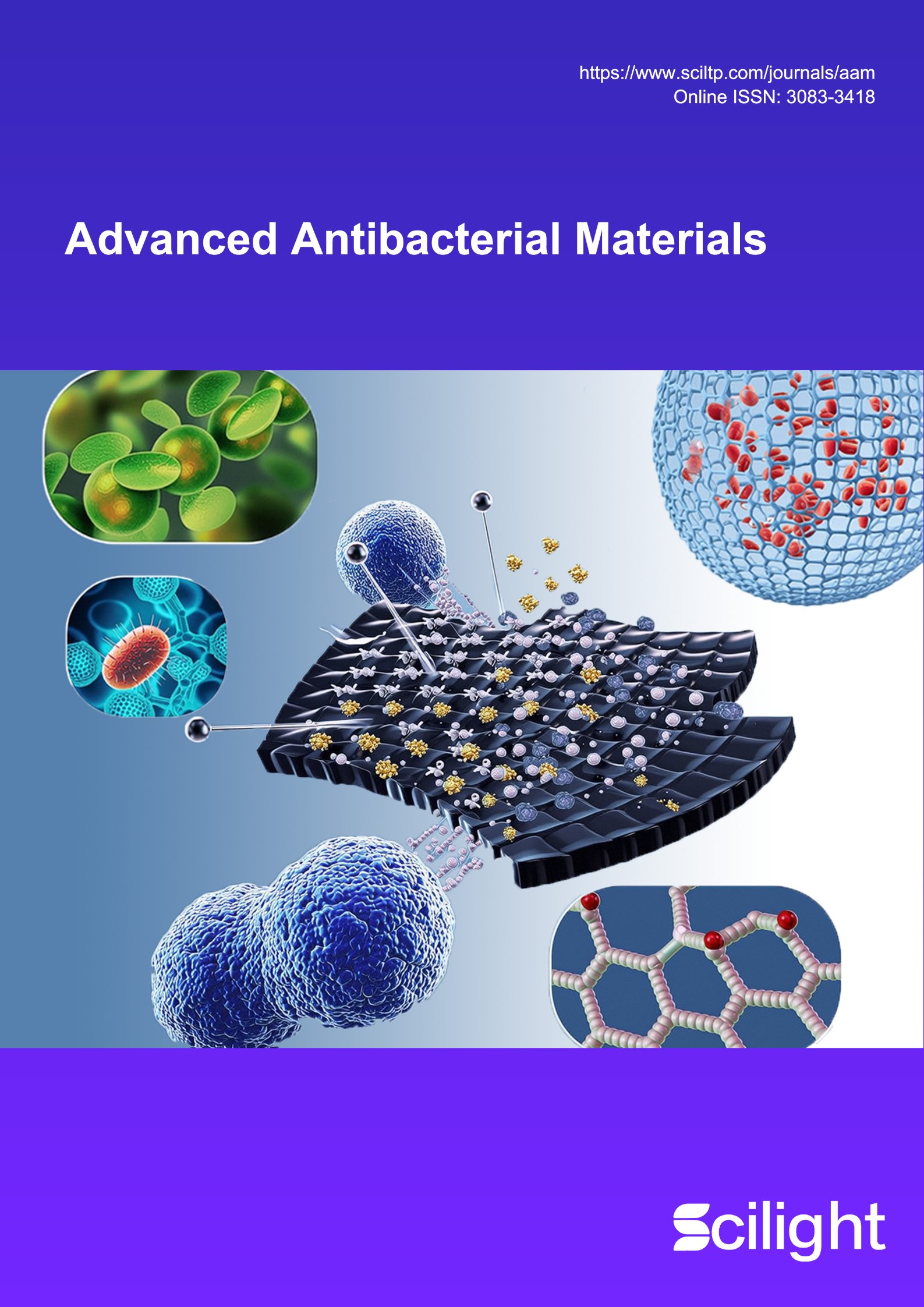
Antimicrobial peptide (AMP) coatings show potential in preventing implant-associated infections (IAI), but their effectiveness is frequently limited by enzymatic degradation and cytotoxicity at elevated densities. This study demonstrates that complete D-amino acid substitution of surface-grafted AMPs effectively addresses these shortcomings. Using a model antimicrobial peptide (DRAMP04195) covalently immobilized on titanium via strain-promoted azide-alkyne cycloaddition (SPAAC), we compared the L- and D-peptide variants. At a high grafting density, Ti-D-80 achieves near-complete eradication of both S. aureus and E. coli (>99.99%), outperforming Ti-L-80. Scanning electron microscopy confirms membrane disruption as the primary antibacterial mechanism for both surfaces. Importantly, Ti-D-80 retains over 90% of its antibacterial activity after protease treatment, whereas Ti-L-80 is almost completely inactivated. Moreover, Ti-D-80 exhibits excellent biocompatibility, supporting cell adhesion and proliferation comparable to that of pristine titanium, in contrast to the cytotoxicity observed with Ti-L-80. These findings establish D-amino acid substitution as an effective strategy to simultaneously enhance enzymatic stability, antibacterial potency, and biocompatibility of AMP-functionalized implants, providing a robust approach for designing durable and tissue-compatible antimicrobial interfaces.
- Open Access
- Article
Titanium Implant Functionalized with D-Amino Acid-Substituted Antimicrobial Peptides for Infection Resistance and Enhanced Biocompatibility
Author Information
Received: 22 Dec 2025 | Revised: 15 Jan 2026 | Accepted: 16 Jan 2026 | Published: 03 Feb 2026
Abstract
Graphical Abstract
Keywords
antimicrobial peptides | D-amino acid substitution | titanium implant | surface grafting | biocompatibility
References
- 1.
Geetha, M.; Singh, A.K.; Asokamani, R.; et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. https://doi.org/10.1016/j.pmatsci.2008.06.004.
- 2.
Moriarty, T.F.; Metsemakers, W.-J.; Morgenstern, M.; et al. Fracture-related infection. Nature Reviews Disease Primers 2022, 8, 67. https://doi.org/10.1038/s41572-022-00396-0.
- 3.
Bozic, K.J.; Kurtz, S.M.; Lau, E.; et al. The Epidemiology of Revision Total Knee Arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45. https://doi.org/10.1007/s11999-009-0945-0.
- 4.
Stewart, P.S.; William Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. https://doi.org/10.1016/S0140-6736(01)05321-1.
- 5.
Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. https://doi.org/10.1038/nrd1008.
- 6.
Kargupta, R.; Bok, S.; Darr, C.M.; et al. Coatings and surface modifications imparting antimicrobial activity to orthopedic implants. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2014, 6, 475–495. https://doi.org/10.1021/10.1002/wnan.1273.
- 7.
Lange, J.; Troelsen, A.; Thomsen, R.W.; et al. Chronic infections in hip arthroplasties: Comparing risk of reinfection following one-stage and two-stage revision: A systematic review and meta-analysis. Clin. Epidemiol. 2012, 4, 57–73. https://doi.org/10.2147/CLEP.S29025.
- 8.
Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; et al. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. https://doi.org/10.1038/s41413-019-0061-z.
- 9.
Yılmaz, Ç.; Özcengiz, G. Antibiotics: Pharmacokinetics, toxicity, resistance and multidrug efflux pumps. Biochem. Pharmacol. 2017, 133, 43–62. https://doi.org/10.1016/j.bcp.2016.10.005.
- 10.
Zhang, H.; Chen, Q.; Xie, J.; et al. Switching from membrane disrupting to membrane crossing, an effective strategy in designing antibacterial polypeptide. Sci. Adv. 2023, 9, eabn0771. https://doi.org/10.1126/sciadv.abn0771.
- 11.
Li, J.; Tan, L.; Liu, X.; et al. Balancing Bacteria–Osteoblast Competition through Selective Physical Puncture and Biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic Acid-Cysteine Nanorods. ACS Nano 2017, 11, 11250–11263. https://doi.org/10.1021/acsnano.7b05620.
- 12.
Zhao, F.; Gao, A.; Liao, Q.; et al. Balancing the Anti-Bacterial and Pro-Osteogenic Properties of Ti-Based Implants by Partial Conversion of ZnO Nanorods into Hybrid Zinc Phosphate Nanostructures. Adv. Funct. Mater. 2024, 34, 2311812. https://doi.org/10.1002/adfm.202311812.
- 13.
Standert, V.; Borcherding, K.; Bormann, N.; et al. Antibiotic-loaded amphora-shaped pores on a titanium implant surface enhance osteointegration and prevent infections. Bioact. Mater. 2021, 6, 2331–2345. https://doi.org/10.1016/j.bioactmat.2021.01.012.
- 14.
Wang, K.; Rong, F.; Peng, H.; et al. Infection Microenvironment-Responsive Coating on Titanium Surfaces for On-Demand Release of Therapeutic Gas and Antibiotic. Adv. Healthc. Mater. 2024, 13, 2304510. https://doi.org/10.1002/adhm.202304510.
- 15.
Wang, K.; Gao, M.; Fan, J.; et al. SrTiO3 Nanotube-Based “Pneumatic Nanocannon” for On-Demand Delivery of Antibacterial and Sustained Osseointegration Enhancement. ACS Nano 2024, 18, 16011–16026. https://doi.org/10.1021/acsnano.4c04478.
- 16.
Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. https://doi.org/10.1038/nrmicro1098.
- 17.
Wang, J.; Dou, X.; Song, J.; et al. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. https://doi.org/10.1002/med.21542.
- 18.
Oliveira Júnior, N.G.; Souza, C.M.; Buccini, D.F.; et al. Antimicrobial peptides: Structure, functions and translational applications. Nat. Rev. Microbiol. 2025, 23, 687–700. https://doi.org/10.1038/s41579-025-01200-y.
- 19.
Chen, R.; Willcox, M.D.P.; Ho, K.K.K.; et al. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. https://doi.org/10.1016/j.biomaterials.2016.01.063.
- 20.
Dong, J.; Chen, F.; Yao, Y.; et al. Bioactive mesoporous silica nanoparticle-functionalized titanium implants with controllable antimicrobial peptide release potentiate the regulation of inflammation and osseointegration. Biomaterials 2024, 305, 122465. https://doi.org/10.1016/j.biomaterials.2023.122465.
- 21.
Li, M.; Bai, J.; Tao, H.; et al. Rational integration of defense and repair synergy on PEEK osteoimplants via biomimetic peptide clicking strategy. Bioact. Mater. 2022, 8, 309–324. https://doi.org/10.1016/j.bioactmat.2021.07.002.
- 22.
He, X.; Zhang, J.; Xie, L.; et al. Phytic Acid-Promoted rapid fabrication of natural polypeptide coatings for multifunctional applications. Chem. Eng. J. 2022, 440, 135917. https://doi.org/10.1016/j.cej.2022.135917.
- 23.
Mohamed, M.F.; Brezden, A.; Mohammad, H.; et al. A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci. Rep. 2017, 7, 6953. https://doi.org/10.1038/s41598-017-07440-0.
- 24.
Hamamoto, K.; Kida, Y.; Zhang, Y.; et al. Antimicrobial Activity and Stability to Proteolysis of Small Linear Cationic Peptides with D-Amino Acid Substitutions. Microbiol. Immunol. 2002, 46, 741–749. https://doi.org/10.1111/j.1348-0421.2002.tb02759.x.
- 25.
McGrath, D.M.; Barbu, E.M.; Driessen, W.H.P.; et al. Mechanism of action and initial evaluation of a membrane active all-D-enantiomer antimicrobial peptidomimetic. Proc. Natl. Acad. Sci. USA 2013, 110, 3477–3482. https://doi.org/10.1073/pnas.1221924110.
- 26.
Lou, T.; Bai, X.; He, X.; et al. Antifouling performance of d-enantiomers-based peptide-modified aluminum alloy surfaces with enhanced stability against proteolytic degradation. J. Mater. Sci. 2023, 58, 15499–15512. https://doi.org/10.1007/s10853-023-08960-z.
- 27.
Shi, G.; Kang, X.; Dong, F.; et al. DRAMP 3.0: An enhanced comprehensive data repository of antimicrobial peptides. Nucleic Acids Res. 2022, 50, D488-D496. https://doi.org/10.1093/nar/gkab651.
- 28.
Kim, S.-J.; Kim, J.-S.; Lee, Y.-S.; et al. Structural Characterization of de Novo Designed L5K5W Model Peptide Isomers with Potent Antimicrobial and Varied Hemolytic Activities. Molecules 2013, 18, 859–876. https://doi.org/10.3390/molecules18010859.
- 29.
Fu, J.; Zhu, W.; Liu, X.; et al. Self-activating anti-infection implant. Nat. Commun. 2021, 12, 6907. https://doi.org/10.1038/s41467-021-27217-4.
- 30.
Park, S.-C.; Kim, J.-Y.; Lee, J.-K.; et al. Synthetic diastereomeric-antimicrobial peptide: Antibacterial activity against multiple drug resistant clinical isolates. Pept. Sci. 2011, 96, 130–136. https://doi.org/10.1002/bip.21446.
- 31.
Personne, H.; Paschoud, T.; Fulgencio, S.; et al. To Fold or Not to Fold: Diastereomeric Optimization of an α-Helical Antimicrobial Peptide. J. Med. Chem. 2023, 66, 7570–7583. https://doi.org/10.1021/acs.jmedchem.3c00460.
- 32.
Wade, D.; Silberring, J.; Soliymani, R.; et al. Antibacterial activities of temporin A analogs. FEBS Lett. 2000, 479, 6–9. https://doi.org/10.1016/S0014-5793(00)01754-3.
- 33.
Loffredo, M.R.; Savini, F.; Bobone, S.; et al. Inoculum effect of antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2021, 118, e2014364118. https://doi.org/10.1073/pnas.2014364118.
- 34.
Huang, Y.; He, L.; Li, G.; et al. Role of helicity of α-helical antimicrobial peptides to improve specificity. Protein Cell 2014, 5, 631–642. https://doi.org/10.1007/s13238-014-0061-0.
- 35.
Chen, H.-L.; Su, P.-Y.; Shih, C. Improvement of in vivo antimicrobial activity of HBcARD peptides by D-arginine replacement. Appl. Microbiol. Biotechnol. 2016, 100, 9125–9132. https://doi.org/10.1007/s00253-016-7621-6.
- 36.
Ng, S.M.S.; Teo, S.W.; Yong, Y.E.; et al. Preliminary investigations into developing all-D Omiganan for treating Mupirocin-resistant MRSA skin infections. Chem. Biol. Drug Des. 2017, 90, 1155–1160. https://doi.org/10.1111/cbdd.13035.
- 37.
Liu, M.; Svirskis, D.; Proft, T.; et al. Progress in peptide and protein therapeutics: Challenges and strategies. Acta Pharm. Sin. B 2025, 15, 6342–6381. https://doi.org/10.1016/j.apsb.2025.10.026.
- 38.
Gellert, M.; Hardt, S.; Köder, K.; et al. Biofilm-active antibiotic treatment improves the outcome of knee periprosthetic joint infection: Results from a 6-year prospective cohort study. Int. J. Antimicrob. Agents 2020, 55, 105904. https://doi.org/10.1016/j.ijantimicag.2020.105904.
- 39.
Caldwell, M.; Hughes, M.; Wei, F.; et al. Promising applications of D-amino acids in periprosthetic joint infection. Bone Res. 2023, 11, 14. https://doi.org/10.1038/s41413-023-00254-z.
- 40.
Duan, S.; Wu, R.; Xiong, Y.-H.; et al. Multifunctional antimicrobial materials: From rational design to biomedical applications. Prog. Mater. Sci. 2022, 125, 100887. https://doi.org/10.1016/j.pmatsci.2021.100887.
- 41.
Oren, Z.; Shai, Y. Selective Lysis of Bacteria but Not Mammalian Cells by Diastereomers of Melittin: Structure−Function Study. Biochemistry 1997, 36, 1826–1835. https://doi.org/10.1021/bi962507l.
- 42.
Arranz-Gibert, P.; Ciudad, S.; Seco, J.; et al. Immunosilencing peptides by stereochemical inversion and sequence reversal: Retro-D-peptides. Sci. Rep. 2018, 8, 6446. https://doi.org/10.1038/s41598-018-24517-6.
- 43.
Yang, J.; Ran, Y.; Liu, S.; et al. Synergistic D-Amino Acids Based Antimicrobial Cocktails Formulated via High-Throughput Screening and Machine Learning. Adv. Sci. 2024, 11, 2307173. https://doi.org/10.1002/advs.202307173.

This work is licensed under a Creative Commons Attribution 4.0 International License.