Efficient ion transport represents a key challenge in the development of solid-state zinc-ion electrolytes. While the introduction of bound water is known to enhance ionic conductivity, the regulation of its content and its precise role in the ion transport mechanism remain inadequately understood. Herein, we systematically investigate the role of water content (0–19.87 wt%) in a poly(ethylene glycol) (PEG)-based viscoelastic solid-state electrolyte (V-SSE) in modulating Zn2⁺ transport and enhancing the performance of Zn||I2 batteries. Through positron annihilation lifetime spectroscopy, Fourier transform infrared spectroscopy, and molecular dynamics simulations, we observe that the free volume expands from 152 Å3 to 163 Å3 with increasing water content up to 9.16%, facilitating ion mobility. Beyond this level, free volume slightly decreases, yet ionic conductivity continues to rise, suggesting alternative promoting mechanisms. At a critical H2O-to-ether oxygen (EO) molar ratio of 1:1, a threefold increase in the ion diffusion coefficient occurs compared to that at H2O:EO = 1:3, stemming from shortened transport distance and enhanced diffusion kinetics. Consequently, the bound water-mediated V-SSE enables a high specific capacity of 170–208 mAh g−1 in Zn||I2 full cells, approximately twice that of the anhydrous system (94 mAh g−1), and maintains stable cycling over 3000 cycles. This study elucidates the multirole mechanism of bound water in enhancing ion conduction without compromising electrochemical stability, providing valuable insights for the design of high-performance solid-state electrolytes.
- Open Access
- Article
Bound Water-Mediated Fast Ion Transport in Viscoelastic Solid-State Electrolyte Boosting Performance of Solid-State Zinc-Ion Batteries
- Yachen Cao 1,
- Weijia Lin 1,
- Lirui Xing 1,
- Minghui Ye 2,
- Yufei Zhang 2,
- Yongchao Tang 2,
- Xiaoqing Liu 2,
- Zhipeng Wen 2,
- Qingyu(Alex) Yan 3,
- Wencheng Du 1, *,
- Chengchao Li 2, *
Author Information
Received: 12 Sep 2025 | Revised: 16 Oct 2025 | Accepted: 18 Oct 2025 | Published: 03 Nov 2025
Abstract
Graphical Abstract
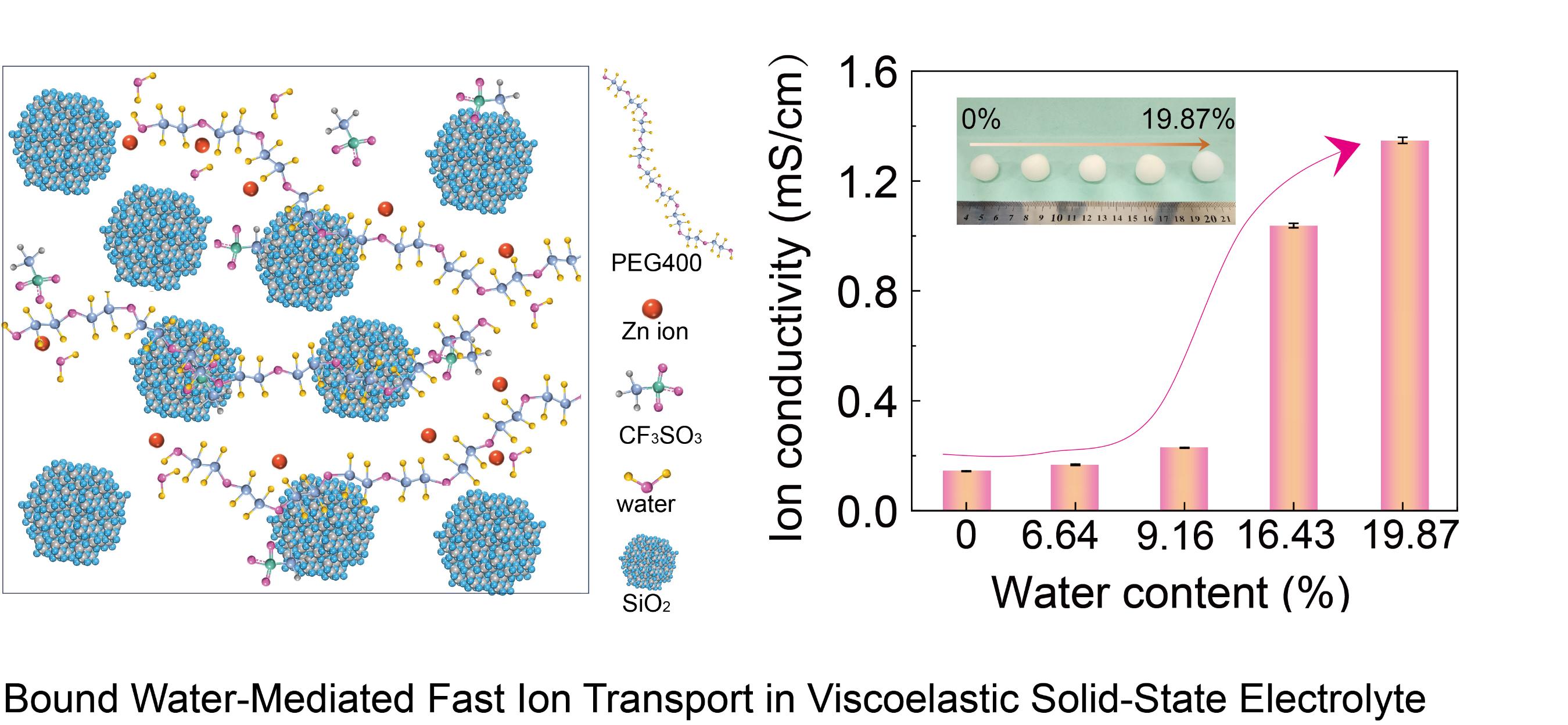
Keywords
solid-state zinc-ion batteries | fast ion transfer | bound water mediation | enlarged free volume | shortened transport distance
References
- 1.Guo, W.; Tian, Y.; Jiang, L. Asymmetric Ion Transport through Ion-Channel-Mimetic Solid-State Nanopores. Acc. Chem. Res. 2013, 46, 2834.
- 2.Farrington, G.C.; Briant, J.L. Fast Ionic Transport in Solids: Crystalline Solids with Liquid-like Ionic Conductivities Are Revolutionizing Solid-State Electrochemistry. Science 1979, 204, 1371.
- 3.Ohno, S.; Banik, A.; Dewald, G.F.; et al. Materials Design of Ionic Conductors for Solid State Batteries. Prog. Energy 2020, 2, 022001.
- 4.Angell, C. Recent Developments in Fast Ion Transport in Glassy and Amorphous Materials. Solid State Ion. 1986, 18–19, 72.
- 5.Chen, R.; Li, Q.; Yu, X.; et al. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chem. Rev. 2020, 120, 6820.
- 6.Thomas, F.; Mahdi, L.; Lemaire, J.; et al. Technological Advances and Market Developments of Solid-State Batteries: A Review. Materials 2024, 17, 239.
- 7.Wang, C.; Sun, X. The Promise of Solid-State Batteries for Safe and Reliable Energy Storage. Engineering 2023, 21, 32.
- 8.Yang, F.; Santos, E.C.D.; Jia, X.; et al. A Dynamic Database of Solid-State Electrolyte (DDSE) Picturing All-Solid-state Batteries. Nano Mater. Sci. 2024, 6, 256.
- 9.Chen, P.; Ding, B.; Dou, H.; et al. Ceramic–Polymer Composite Solid-State Electrolytes for Solid-State Lithium Metal Batteries: Mechanism, Strategy, and Prospect. Small 2025, 2503743.
- 10.Yu, T.; Liu, Y.; Li, H.; et al. Ductile Inorganic Solid Electrolytes for All-Solid-State Lithium Batteries. Chem. Rev. 2025, 125, 3595.
- 11.Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring Inorganic–Polymer Composites for the Mass Production of Solid-State Batteries. Nat. Rev. Mater. 2021, 6, 1003.
- 12.Wu, J.; Chen, W.; Hao, B.; et al. Garnet-Type Solid-State Electrolytes: Crystal-Phase Regulation and Interface Modification for Enhanced Lithium Metal Batteries. Small 2025, 21, 2407983.
- 13.Zhao, Z.; Wang, J.; Lv, Z.; et al. In-situ Formed All-amorphous Poly (ethylene oxide)-based Electrolytes Enabling Solid-State Zn Electrochemistry. Chem. Eng. J. 2021, 417, 128096.
- 14.Dai, T.; Wu, S.; Lu, Y.; et al. Inorganic Glass Electrolytes with Polymer-like Viscoelasticity. Nat. Energy 2023, 8, 1221.
- 15.Jian, S.; Cao, Y.; Feng, W.; et al. Recent Progress in Solid Polymer Electrolytes with Various Dimensional Fillers: A Review. Mater. Today Sustain. 2022, 20, 100224.
- 16.Xue, S.; Chen, S.; Fu, Y.; et al. Revealing the Role of Active Fillers in Li-ion Conduction of Composite Solid Electrolytes. Small 2023, 19, 2305326.
- 17.Liu, J.; Wang, T.; Yu, J.; et al. Review of the Developments and Difficulties in Inorganic Solid-State Electrolytes. Materials 2023, 16, 2510.
- 18.Xiao, Y.; Wang, Y.; Bo, S.-H.; et al. Understanding Interface Stability in Solid-State Batteries. Nat. Rev. Mater. 2019, 5, 105.
- 19.Zhang, S.; Long, T.; Zhang, H.; et al. Electrolytes for Multivalent Metal-Ion Batteries: Current Status and Future Prospect. ChemSusChem 2022, 15, e202200999.
- 20.Raza, S.; Bashir, T.; Hayat, A.; et al. Recent Progress and Fundamentals of Solid-State Electrolytes for All Solid-State Rechargeable Batteries: Mechanisms, Challenges, and Applications. J. Energy Storage 2024, 92, 112110.
- 21.O’Donnell, L.F.; Greenbaum, S.G. Review of Multivalent Metal Ion Transport in Inorganic and Solid Polymer Electrolytes. Batteries 2020, 7, 3.
- 22.Li, R.; Deng, R.; Wang, Z.; et al. The Challenges and Perspectives of Developing Solid-State Electrolytes for Rechargeable Multivalent Battery. J. Solid State Electrochem. 2023, 27, 1291.
- 23.Jeschull, F.; Hub, C.; Kolesnikov, T.I.; et al. Multivalent Cation Transport in Polymer Electrolytes—Reflections on an Old Problem. Adv. Energy Mater. 2024, 14, 2302745.
- 24.Tian, W.; Lin, G.; Yuan, S.; et al. Competitive Coordination and Dual Interphase Regulation of MOF-Modified Solid-State Polymer Electrolytes for High-Performance Sodium Metal Batteries. Angew. Chem. Int. Ed. 2025, 64, e202423075.
- 25.Jones, S.D.; Bamford, J.; Fredrickson, G.H.; et al. Decoupling Ion Transport and Matrix Dynamics to Make High Performance Solid Polymer Electrolytes. ACS Polym. Au 2022, 2, 430.
- 26.Shinde, S.S.; Wagh, N.K.; Kim, S.; et al. Li, Na, K, Mg, Zn, Al, and Ca Anode Interface Chemistries Developed by Solid-State Electrolytes. Adv. Sci. 2023, 10, 2304235.
- 27.McAlpine, J.; Bloemendal, A.; Dahl, J.E.; et al. Modulating Entropic Driving Forces to Promote High Lithium Mobility in Solid Organic Electrolytes. Chem. Mater. 2023, 35, 3545.
- 28.Hou, Y.; Wei, Z.; Wu, Z.; et al. Regulating Dielectricity of A Polymer Electrolyte to Promote Cation Mobility for High-Performance Solid Zinc Hybrid Batteries. Energy Environ. Sci. 2024, 17, 3917.
- 29.Wang, J.; Zhao, Z.; Lu, G.; et al. Room-temperature Fast Zinc-Ion Conduction in Molecule-Flexible Solids. Mater. Today Energy 2021, 20, 100630.
- 30.Miao, C.; Wang, X.; Guan, D.; et al. Spatially Confined Engineering Toward Deep Eutectic Electrolyte in Metal-Organic Framework Enabling Solid-State Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202410208.
- 31.Yan, S.; Lu, Y.; Liu, F.; et al. Zwitterionic Matrix with Highly Delocalized Anionic Structure as an Efficient Lithium Ion Conductor. CCS Chem. 2023, 5, 1612.
- 32.Vélez, J.F.; Aparicio, M.; Mosa, J. Effect of Lithium Salt in Nanostructured Silica–Polyethylene Glycol Solid Electrolytes for Li-Ion Battery Applications. J. Phys. Chem. C 2016, 120, 22852.
- 33.Di Noto, V.; Longo, D.; Münchow, V. Ion-Oligomer Interactions in Poly(ethylene glycol)400/(LiCl)x Electrolyte Complexes. J. Phys. Chem. B 1999, 103, 2636.
- 34.Aurbach, D.; Lu, Z.; Schechter, A.; et al. Prototype Systems for Rechargeable Magnesium Batteries. Nature 2000, 407, 724.
- 35.Lin, W.; Zhou, K.; Xing, L.; et al. Viscoelastic Soft Solid Electrolytes Enable Fast Zinc Ion Conductance and Highly Stable Zinc Metal Anode. Adv. Energy Mater. 2025, 15, 2404545.
- 36.Martínez, L.; Andrade, R.; Birgin, E.G.; et al. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157.
- 37.Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; et al. Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides. J. Phys. Chem. B 2001, 105, 6474.
- 38.Abraham, M.J.; Murtola, T.; Schulz, R.; et al. GROMACS: High Performance Molecular Simulations Through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19.
- 39.Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33.
- 40.Pas, S.J.; Ingram, M.D.; Funke, K.; et al. Free Volume and Conductivity in Polymer Electrolytes. Electrochimica Acta 2005, 50, 3955.
- 41.Miyamoto, T.; Shibayama, K. Free-Volume Model for Ionic Conductivity in Polymers. J. Appl. Phys. 1973, 44, 5372.
- 42.Bamford, D.; Dlubek, G.; Reiche, A.; et al. The Local Free Volume, Glass Transition, and Ionic Conductivity in A Polymer Electrolyte: A Positron Lifetime Study. J. Chem. Phys. 2001, 115, 7260.
- 43.Halat, D.M.; Snyder, R.L.; Sundararaman, S.; Choo, Y.; et al. Modifying Li+ and Anion Diffusivities in Polyacetal Electrolytes: A Pulsed-Field-Gradient NMR Study of Ion Self-Diffusion+. Chem. Mater. 2021, 33, 4915.
- 44.Kost, B.; Basko, M.; Kaźmierski, S.; et al. Polyacetals of Higher Cyclic Formals: Synthesis, Properties and Application as Polymer Electrolytes. Polym. Chem. 2025, 16, 598.
- 45.Ramya, P.; Ranganathaiah, C.; Williams, J.F. Experimental Determination of Interface Widths in Binary Polymer Blends from Free Volume Measurements. Polymer 2012, 53, 4539.
- 46.Mor, J.; Pandey, K.L.; Sharma, S.K. Correlation Between Free Volume Structure and Ionic Conductivity of A Poly(ethylene oxide) and Dendritic Fibrous Nanosilica Composite-based Electrolyte: An Investigation Using Positron Annihilation and Broadband Dielectric Spectroscopy. Phys. Chem. Chem. Phys. 2025, 27, 10082.
- 47.Utpalla, P.; Sharma, S.K.; Sudarshan, K.; et al. Free Volume Correlation with AC Conductivity and Thermo-Mechanical Properties of Poly (ethylene oxide)-silica Nanocomposites. Eur. Polym. J. 2019, 117, 10.
- 48.Zhan, Y.; Fu, W.; Xing, Y.; et al. Advances in Versatile Anti-Swelling Polymer Hydrogels. Mater. Sci. Eng. C 2021, 127, 112208.
- 49.Brogly, M.; Bistac, S.; Bindel, D. Adsorption and Structuration of PEG Thin Films: Influence of the Substrate Chemistry. Polymers 2024, 16, 1244.
- 50.Rocco, A.M.; Moreira, D.P.; Pereira, R.P. Specific Interactions in Blends of Poly(ethylene oxide) and Poly(bisphenol A-co-epichlorohydrin): FTIR and Thermal Study. Eur. Polym. J. 2003, 39, 1925.
- 51.Brooks, D.J.; Merinov, B.V.; Goddard, W.A.; et al. Atomistic Description of Ionic Diffusion in PEO–LiTFSI: Effect of Temperature, Molecular Weight, and Ionic Concentration. Macromolecules 2018, 51, 8987.
- 52.Gao, Y.; Nolan, A.M.; Du, P.; et al. Classical and Emerging Characterization Techniques for Investigation of Ion Transport Mechanisms in Crystalline Fast Ionic Conductors. Chem. Rev. 2020, 120, 5954.
- 53.He, X.; Zhu, Y.; Mo, Y. Origin of Fast Ion Diffusion in Super-Ionic Conductors. Nat. Commun. 2017, 8, 15893.
- 54.Sharon, D.; Deng, C.; Bennington, P.; et al. Critical Percolation Threshold for Solvation-Site Connectivity in Polymer Electrolyte Mixtures. Macromolecules 2022, 55, 7212.
- 55.Yang, X.; Jiang, M.; Gao, X.; et al. Determining the Limiting Factor of the Electrochemical Stability Window for PEO-based Solid Polymer Electrolytes: Main Chain or Terminal –OH Group? Energy Environ. Sci. 2020, 13, 1318.
- 56.Rong, Z.; Sun, Y.; Yang, M.; et al. How the PEG Terminals Affect the Electrochemical Properties of Polymer Electrolytes in Lithium Metal Batteries. Energy Storage Mater. 2023, 63, 103066.
- 57.Yan, K.; Fan, Y.; Hu, F.; et al. A “Polymer-in-Salt” Solid Electrolyte Enabled by Fast Phase Transition Route for Stable Zn Batteries. Adv. Funct. Mater. 2024, 34, 2307740.
- 58.Lv, Z.; Kang, Y.; Chen, J.; et al. Stable Solid-State Zinc–Iodine Batteries Enabled by an Inorganic ZnPS3 Solid Electrolyte with Interconnected Zn2+ Migration Channels. Adv. Funct. Mater. 2024, 34, 2310476.
- 59.Zhou, C.; Wang, Z.; Nan, Q.; et al. Simultaneous Inhibition of Vanadium Dissolution and Zinc Dendrites by Mineral-Derived Solid-State Electrolyte for High-Performance Zinc Metal Batteries. Angew. Chem. Int. Ed. 2024, 136, e202412006.
- 60.Chen, Z.; Huang, Z.; Wang, C.; et al. Supramolecular Crystals based Fast Single Ion Conductor for Long-Cycling Solid Zinc Batteries. Angew. Chem. Int. Ed. 2024, 63, e202406683.
How to Cite
Cao, Y.; Lin, W.; Xing, L.; Ye, M.; Zhang, Y.; Tang, Y.; Liu, X.; Wen, Z.; Yan, Q.; Du, W.; Li, C. Bound Water-Mediated Fast Ion Transport in Viscoelastic Solid-State Electrolyte Boosting Performance of Solid-State Zinc-Ion Batteries. eChem 2025, 1 (1), 4. https://doi.org/10.53941/echem.2025.100004.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


