The intensive use of chemical fertilizers has boosted agricultural yields but caused severe environmental concerns, including soil degradation, water pollution, and greenhouse gas emissions. Sustainable alternatives are therefore urgently needed. Insect frass, a nutrient-rich by-product of insect farming, and microalgae, with their ability to produce phytohormones and improve soil quality, have both been proposed as promising biofertilizers. While their individual applications are well documented, little attention has been given to their combined use. This review provides an updated synthesis of current knowledge on insect frass composition, the agronomic value of microalgae, and the first experimental evidence on the use of frass as a nutrient source for microalgal cultivation. Benefits and challenges are discussed, including nutrient variability, microbial safety, heavy metal accumulation, and production costs. The integrated perspective offered here highlights the potential of frass–microalgae systems to support circular bioeconomy models and reduce reliance on chemical fertilizers.
- Open Access
- Review
Insect Frass as a Sustainable Medium for Microalgae Growth: Bridging Waste Valorization and Eco-Physiological Innovation
- Federica Impellitteri 1,
- Giorgia Cannatà 2,
- Emanuela Castronovo 2,
- Shubhajit Saha 3,
- Caterina Faggio 2,4,*,
- Cristiana Roberta Multisanti 1,
- Maria Giovanna Rizzo 2
Author Information
Received: 06 Nov 2025 | Revised: 17 Dec 2025 | Accepted: 22 Dec 2025 | Published: 04 Jan 2026
Abstract
Graphical Abstract
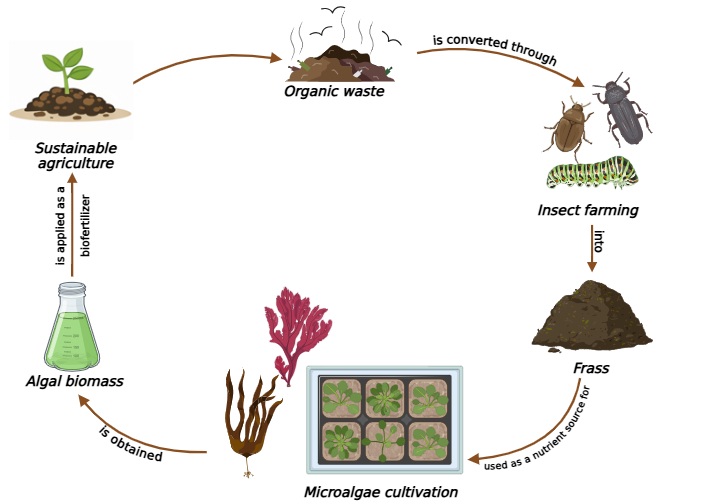
Keywords
circular bioeconomy | sustainable agriculture | waste valorization | biofertilizer | microalgae | frass
References
- 1.
Khan, Z.; Ali, S.; Chu, X. Environmental sustainability via sustainable development: The role of green innovation, clean energy and financial inclusion. J. Environ. Manag. 2025, 393, 127175. https://doi.org/10.1016/j.jenvman.2025.127175.
- 2.
Teixeira, N.; Rodrigues, R.; Rodrigues, A. Economic growth and environmental sustainability in more and less sustainable countries. Discov. Sustain. 2025, 6, 618. https://doi.org/10.1007/s43621-025-01546-6.
- 3.
Gonçalves, J.; Freitas, J.; Fernandes, I.; et al. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability 2023, 15, 12413. https://doi.org/10.3390/su151612413.
- 4.
Lopes, G.I.; Yong, W.H.J.; Lalander, C. Frass derived from black soldier fly larvae treatment of biodegradable wastes. A critical review and future perspectives. J. Waste Manag. 2022, 142, 65–76. https://doi.org/10.1016/j.wasman.2022.02.007.
- 5.
Leni, G.; Caligiani, A.; Sforza, S. Chapter 40-Bioconversion of Agri-Food Waste and by-Products through Insects: A New Valorization Opportunity. In Valorization of Agri-Food Wastes and By-Products; Academic Press: Cambridge, MA, USA, 2021; pp. 809–828. https://doi.org/10.1016/B978-0-12-824044-1.00013-1.
- 6.
Magara, J.O.H.; Tanga, C.M.; Ayieko, M.A.; et al. Effect of rearing substrates on the fitness parameters of newly recorded edible cricket Scapsipedus marginatus in Kenya. In Proceedings of the Second International Conference ‘Insects to feed the world’ (IFW2018), Wuhan, China, 15–18 May 2018.
- 7.
Gómez-Brandón, M.; Beesigamukama, D.; Probst. M.; et al. Microbial composition and bioremediation in frass fertilizers from insect-based agri-food waste valorization. J. Environ. Manag. 2025, 386, 125774. https://doi.org/10.1016/j.jenvman.2025.125774.
- 8.
Khedr, M.M.A.; El-Shafiey, S.N.; Mead, H.M. Influence of fortification of mulberry leaves with natural and synthetic multivitamins on growth and development of Bombyx mori L. J. Plant Prot. Pathol. 2013, 4, 111–123.
- 9.
Leonardi, A.A.; Sciuto, E.L.; Lo Faro, M.J.; et al. Molecular Fingerprinting of the Omicron Variant Genome of SARS-CoV-2 by SERS Spectroscopy. Nanomaterials 2022, 12, 2134. https://doi.org/10.3390/nano12132134.
- 10.
Barsati, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal. Res. 2018, 31, 107–115. https://doi.org/10.1016/j.algal.2018.02.001.
- 11.
Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; PizañaAranda, J.J.P.; et al. Microalgae-Based Biotechnology as Alternative Biofertilizers for Soil Enhancement and Carbon Footprint Reduction: Advantages and Implications. Mar. Drugs 2023, 21, 93. https://doi.org/10.3390/md21020093.
- 12.
Petrucciani, A.; Mollo, L.; Siena, S.A.; et al. From waste to products: Microalgal cultivation in insect frass to obtain valuable biomass. Bioresour. Technol. Rep. 2025, 32, 102339. https://doi.org/10.1016/j.biteb.2025.102339.
- 13.
Multisanti, C.R.; Impellitteri, F.; Cannatà, G.; et al. Discovering the effects of octylisothiazolinone: Analysis of physiological changes in the Mediterranean mussel (Mytilus galloprovincialis). Ecotoxicol. Environ. Saf. 2025, 302, 118563. https://doi.org/10.1016/j.ecoenv.2025.118563.
- 14.
Riolo, K.; Rotondo, A.; La Torre, G.L.; et al. Cytoprotective and antioxidant effects of hydrolysates from black soldier fly (Hermetia illucens). Antioxidants 2023, 12, 519. https://doi.org/10.3390/antiox12020519.
- 15.
Siddiqui, S.A.; Harahap, I.A.; Osei-Owusu, J.; et al. Bioconversion of organic waste by insects—A comprehensive review. Process Saf. Environ. Prot. 2024, 187, 1–25. https://doi.org/10.1016/j.psep.2024.04.122.
- 16.
Beesigamukama, D.; Tanga, M.C.; Sevgan, S.; et al. Waste to value: Global perspective on the impact of entomocomposting on environmental health, greenhouse gas mitigation and soil bioremediation. Sci. Total Environ. 2023, 902, 166067. https://doi.org/10.1016/j.scitotenv.2023.166067.
- 17.
Magara, H.J.; Tanga, C.M.; Ayieko, M.A.; et al. Performance of newly described native edible cricket Scapsipedus icipe (Orthoptera: Gryllidae) on various diets of relevance for farming. J. Econ. Entomol. 2019, 112, 653–664.
- 18.
Lomonaco, G.; Franco, A.; De Smet, J.; et al. Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops. Insects 2024, 15, 293. https://doi.org/10.3390/insects15040293.
- 19.
Amorim, H.C.S.; Ashworth, A.J.; Arsi, K; et al. Insect frass composition and potential use as an organic fertilizer in circular economies. J. Econ. Entomol. 2024, 117, 1261–1268. https://doi.org/10.1093/jee/toad234.
- 20.
De Volder, A.; De Smet, J.; Frooninckx, L.; et al. Heat Treatment and Storage of Frass from Black Soldier Fly Larvae and Yellow Mealworm Production: Compliance with EU Regulation on Microbiological Quality and Safety. MicrobiologyOpen 2025, 14, e70020. https://doi.org/10.1002/mbo3.70020.
- 21.
Lalander, C.; Lopes, I.G.; Gyftopoulos, N.; et al. The impact of scale and frass recirculation on pathogen inactivation dynamics in black soldier fly larvae bioconversion. Front. Microbiol. 2025, 16, 1539486. https://doi.org/10.3389/fmicb.2025.1539486.
- 22.
Athanassiou, C.G.; Rumbos, C.I. Frass and Furious: Unfolding the Potential of Insect Frass as Soil Fertilizer. Agrochemicals 2025, 4, 1. https://doi.org/10.3390/agrochemicals4010001.
- 23.
Houben, D.; Daoulas, G.; Faucon, M.-P.; et al. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020, 10, 4659. https://doi.org/10.1038/s41598-020-61765-x.
- 24.
Beesigamukama, D.; Mochoge, B.; Korir, N.; et al. Economic and ecological values of frass fertiliser from black soldier fly agro-industrial waste processing. J. Insects Food Feed. 2022, 8, 245–254. https://doi.org/10.3920/JIFF2021.0013.
- 25.
Praeg, N.; Klammsteiner, T. Primary study on frass fertilizers from mass-reared insects: Species variation, heat treatment effects, and implications for soil application at laboratory scale. J. Environ. Manag. 2024, 356, 120622. https://doi.org/10.1016/j.jenvman.2024.120622.
- 26.
Van Looveren, N.; Vandeweyer, D.; Van Campenhout, L. Impact of Heat Treatment on the Microbiological Quality of Frass Originating from Black Soldier Fly Larvae (Hermetia illucens). Insects 2022, 13, 22. https://doi.org/10.3390/insects13010022.
- 27.
Beesigamukama, D.; Mochoge, B.; Korir, N.K.; et al. Exploring Black Soldier Fly Frass as Novel Fertilizer for Improved Growth, Yield, and Nitrogen Use Efficiency of Maize Under Field Conditions. Front. Plant Sci. 2020, 11, 574592. https://doi.org/10.3389/fpls.2020.574592.
- 28.
Ashworth, A.J.; Amorim, H.C.S.; Drescher, G.L.; et al. Insect frass fertilizer as soil amendment for improved forage and soil health in circular systems. Sci. Rep. 2025, 15, 3024. https://doi.org/10.1038/s41598-025-87075-8.
- 29.
Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient quality and maturity status of frass fertilizer from nine edible insects. Sci. Rep. 2022, 12, 7182. https://doi.org/10.1038/s41598-022-11336-z.
- 30.
Mutyambai, D.M.; Mutua, J.M.; Jalloh, A.A.; et al. Insect frass fertilizer upregulates maize defence genes and resistance against an invasive herbivore pest. Sci. Rep. 2025, 15, 29978. https://doi.org/10.1038/s41598-025-14883-3.
- 31.
Salomon, M.J.; Cavagnaro, T.R. Potential of black soldier fly larvae frass (BSFL) as a novel fertilizer: Impacts on tomato growth, nutrient uptake, and mycorrhizal formation. Plant Soil. 2025, 513, 417–434 https://doi.org/10.1007/s11104-024-07187-4.
- 32.
Sawinska, Z.; Radzikowska-Kujawska, D.; Kowalczewski, P.Ł.; et al. Hermetia illucens Frass Fertilization: A Novel Approach for Enhancing Lettuce Resilience and Photosynthetic Efficiency under Drought Stress Conditions. Appl. Sci. 2024, 14, 2386. https://doi.org/10.3390/app14062386.
- 33.
Rizzo, M.G.; Corsaro, C.; Marrara, S.; et al. Raman spectral analyses to investigate the physiological and metabolic development of a 3D hepatocellular carcinoma model. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 343, 126564. https://doi.org/10.1016/j.saa.2025.126564.
- 34.
Multisanti, C.R.; Zicarelli, G.; Caferro, A.; et al. Personal care products as a potential source of aquatic pollution: Effect of polyvinyl alcohol on physiological and antioxidant responses in Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2025, 298, 118336. https://doi.org/10.1016/j.ecoenv.2025.118336.
- 35.
Multisanti, C.R.; Riolo, K.: Rizzo, M.G.; et al. Novel sustainable strategies to mitigate toxicity of emerging contaminants: Cellular and physiological insights from CMIT-exposed mussels treated with insect-based protein hydrolysates. Sustain. Chem. Pharm. 2025, 48, 102218. https://doi.org/10.1016/j.scp.2025.102218.
- 36.
Albalawneh, A.; Hasan, H.; Alarsan, S.F.; et al. Influence of Sludge and Feed Mixtures on Metal Retention, Pathogen Reduction, and Nutritional Value in Black Soldier Fly (BSF) (Hermetia illucens) Larval Substr. Agriculture 2025, 15, 1080. https:// doi.org/10.3390/agriculture15101080.
- 37.
Zunzunegui, I.; Martín-García, J.; Santamaría, Ó.; et al. Insect Frass as an Agricultural Resource Against Abiotic and Biotic Crop Stresses: Mechanisms of Action and Possible Negative Effects. Appl. Sci. 2025, 15, 3606. https://doi.org/10.3390/app15073606.
- 38.
TechSci Research. Available online: https://www.techsciresearch.com/report/frass-fertilizer-market/15817.html (accessed on 20 October 2025).
- 39.
Abreu, A.P.; Martins, R.; Nunes, J. Emerging applications of Chlorella sp. and Spirulina (Arthrospira) sp. Bioengineering 2023, 10, 955. https://doi.org/10.3390/bioengineering10080955.
- 40.
Dolganyuk, V.; Belova, D.; Babich, O.; et al. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. https://doi.org/10.3390/biom10081153.
- 41.
Srimongkol, P.; Sangtanoo, P.; Songserm, P.; et al. Microalgae-based wastewater treatment for developing economic and environmental sustainability: Current status and future prospects. Front. Bioeng. Biotechnol. 2022, 10, 904046. https://doi.org/10.3389/fbioe.2022.904046.
- 42.
Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; et al. Comparison between Microalgal Autotrophic Growth and Metabolite Accumulation with Heterotrophic, Mixotrophic and Photoheterotrophic Cultivation Modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. https://doi.org/10.1016/j.rser.2022.112247.
- 43.
Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs. 2019, 17, 304. https://doi.org/10.3390/md17050304.
- 44.
Champenois, J.; Marfaing, H.; Pierre, R. Review of the Taxonomic Revision of Chlorella and Consequences for Its Food Uses in Europe. J. Appl. Phycol. 2015, 27, 1845–1851. https://doi.org/10.1007/s10811-014-0431-2.
- 45.
Sili, C.; Torzillo, G.; Vonshak, A. Arthrospira (Spirulina). In Ecology of Cyanobacteria II. Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; p. 760. https://doi.org/10.1007/978-94-007-3855-3.
- 46.
Banaee, M.; Impellitteri, F.; Evaz-Zadeh Samani, H.; et al. Dietary arthrospira platensis in rainbow trout (Oncorhynchus mykiss): A means to reduce threats caused by CdCl2 exposure? Toxics 2022, 10, 731. https://doi.org/10.3390/toxics10120731.
- 47.
Coppens, J.; Grunert, O.; Van Den Hende, S.; et al. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. https://doi.org/10.1007/s10811-015-0775-2.
- 48.
Alobwede, E.; Leake, J.R.; Pandhal, J. Circular economy fertilization: Testing micro and macro algal species as soil improvers and nutrient sources for crop production in greenhouse and field conditions. Geoderma 2019, 334, 113–123. https://doi.org/10.1016/j.geoderma.2018.07.049.
- 49.
El Sabagh, A.; Islam, M.S.; Hossain, A. et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. https://doi.org/10.3389/fagro.2022.765068.
- 50.
Tarraf, S.A.; Talaat, I.M.; El-Sayed, A.E.K.B.; et al. Influence of foliar application of algae extract and amino acids mixture on fenugreek plants in sandy and clay soils. Nusant. Biosci. 2015, 7, 32–37. https://doi.org/10.13057/nusbiosci/n070106.
- 51.
Han, X.; Zeng, H.; Bartocci, P.; et al. Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation 2018, 4, 25. https://doi.org/10.3390/fermentation4020025.
- 52.
Kozlova, T.A.; Hardy, B.P.; Krishna, P.; et al. Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Res. 2017, 27, 325–334. https://doi.org/10.1016/j.algal.2017.09.020.
- 53.
Romanenko, E.A.; Kosakovskaya, I.V.; Romanenko, P.O. Phytohormones of microalgae: Biological role and involvement in the regulation of physiological processes. Pt I. auxins, abscisic acid, ethylene. Int. J. Algal 2015, 17, 275–289. https://doi.org/10.1615/InterJAlgae.v17.i3.80.
- 54.
Went, F.; Thimann, K. Phytohormones; The Macmillan Company: New York, NY, USA, 1937.
- 55.
Romanenko, K.O.; Kosakovskaya, I.V.; Romanenko, P.O. Phytohormones of Microalgae: Biological Role and Involvement in the Regulation of Physiological Processes. Pt II. Cytokinins and Gibberellins. Int. J. Algae 2016, 18, 179–201. https://doi.org/10.1615/InterJAlgae.v18.i2.70.
- 56.
Piotrowska, A.; Czerpak, R. Cellular response of light/dark-grown green alga Chlorella vulgaris Beijerinck (Chlorophyceae) to exogenous adenine-and phenylurea-type cytokinins. Acta Plant Physiol. 2009, 31, 573–585. https://doi.org/10.1007/s11738-008-0267-y.
- 57.
Stirk, W.A.; Bálint, P., Tarkowská, D.; et al. Effect of light on growth and endogenous hormones in Chlorella minutissima (Trebouxiophyceae). Plant Physiol. Biochem. 2014, 79, 66–76. https://doi.org/10.1016/j.plaphy.2014.03.005.
- 58.
Du, K.; Tao, H.; Wen, X.; et al. Enhanced growth and lipid production of Chlorella pyrenoidosa by plant growth regulator GA3. Fresenius Environ. Bull. 2015, 24, 3414–3419.
- 59.
Parsaeimehr, A.; Mancera-Andrade, E.I.; Robledo-Padilla, F.; et al. A chemical approach to manipulate the algal growth, lipid content and high-value alpha-linolenic acid for biodiesel production. Algal Res. 2017, 26, 312–322. https://doi.org/10.1016/j.algal.2017.08.016.
- 60.
Wu, G.; Gao, Z.; Du, H.; et al. The effects of abscisic acid, salicylic acid and jasmonic acid on lipid accumulation in two freshwater Chlorella strains. J. Gen. Appl. Microbiol. 2018, 64, 42–49. https://doi.org/10.2323/jgam.2017.06.001.
- 61.
Bayrak, M.; Yüce, P.A.; Günal, A.Ç.; et al. Physiological and histopathological response of non-target organisms to emamectin benzoate: An effective innovative insecticide or a new ecosystem health hazard? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 296, 110241. https://doi.org/10.1016/j.cbpc.2025.110241.
- 62.
Impellitteri, F.; Mossotto, C.; Cotugno, A.; et al. When crayfish face painkillers: Tissue-specific cytotoxic and oxidative responses to indomethacin exposure in Procambarus clarkii. Ecotoxicol. Environ. Saf. 2025, 304, 119138. https://doi.org/10.1016/j.ecoenv.2025.119138.
- 63.
Trivedi, A.; Saxena, V.; Banaee, M.; et al. Unveiling the crosstalk between unfolded protein response and apoptosis in triclosan induced hepatotoxicity in Labeo rohita. Sci. Rep. 2025, 15, 17089. https://doi.org/10.1038/s41598-025-93997-0.
- 64.
Burgos-Aceves, M.A.; Banaee, M.; Vazzana, I.; et al. Effect of emerging pollutants on the gut microbiota of freshwater animals: Focusing on microplastics and pesticides. Sci. Total Environ. 2024, 948, 174809. https://doi.org/10.1016/j.scitotenv.2024.174809.
- 65.
Hossinian, M.; Banaee, M.; Haghi, B.N.; et al. Individual and combined effects of dichlorvos and nano-polystyrene on oxidative stress and biochemical parameters in the freshwater fish Barbus sharpeyi. Emerg. Contam. 2025, 11, 100586. https://doi.org/10.1016/j.emcon.2025.100586.
- 66.
Jindal, R.; Sharma, R.; Kaur, P.; et al. Mitigation of haemato-genotoxic and stress response effects in Cyprinus carpio via silymarin dietary supplementation following deltamethrin exposure. Heliyon 2024, 10, e28419. https://doi.org/10.1016/j.heliyon.2024.e28419.
- 67.
Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; et al. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. https://doi.org/10.1016/j.algal.2021.102200.
- 68.
González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; et al. Microalgae as Biostimulants: A New Approach in Agriculture. World J. Microbiol. Biotechnol. 2022, 38, 4. https://doi.org/10.1007/s11274-021-03192-2.
- 69.
Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. https://doi.org/10.3390/app11020871.
- 70.
Ramya, S.; Barathinivas, A.; Jayakumararaj, R.; et al. Ecotoxicological insights: Effects of pesticides on ionic metabolism regulation in freshwater catfish, Mystus keletius. Aquat. Toxicol. 2023, 265, 106764. https://doi.org/10.1016/j.aquatox.2023.106764.
- 71.
Barathinivas, A.; Ramya, S.; Neethirajan, K.; et al. Ecotoxicological effects of pesticides on hematological parameters and oxidative enzymes in freshwater Catfish, Mystus keletius. Sustainability 2022, 14, 9529. https://doi.org/10.3390/su14159529.
- 72.
Vajargah, M.F.; Namin, J.I.; Mohsenpour, R.; et al. Histological effects of sublethal concentrations of insecticide Lindane on intestinal tissue of grass carp (Ctenopharyngodon idella). Vet. Res. Commun. 2021, 45, 373–380. https://doi.org/10.1007/s11259-021-09818-y.
- 73.
Stara, A.; Bellinvia, R.; Velisek, J.; et al. Acute exposure of common yabby (Cherax destructor) to the neonicotinoid pesticide. Sci. Total Environ. 2019, 665, 718–723. https://doi.org/10.1016/j.scitotenv.2019.02.202.
- 74.
Sula, E.; Aliko, V.; Marku, E.; et al. Evaluation of kidney histopathological alterations in Crucian Carp, Carassius carassius, from a pesticide and PCB-contaminated freshwater ecosystem, using light microscopy and organ index mathematical model. Int. J. Aquat. Biol. 2020, 8, 154–165.
- 75.
Rizzo, C.; Zammuto, V.; Lo Giudice, A.; et al. Antibiofilm activity of antarctic sponge-associated bacteria against pseudomonas aeruginosa and staphylococcus aureus. J. Mar. Sci. Eng. 2021, 9, 243. https://doi.org/10.3390/jmse9030243.
- 76.
Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. https://doi.org/10.3389/fmicb.2018.01636.
- 77.
Rocha, F.; Esteban Lucas-Borja, M.; Pereira, P.; et al. Cyanobacteria as a Nature-Based Biotechnological Tool for Restoring Salt-Affected Soils. Agronomy 2020, 10, 1321. https://doi.org/10.3390/agronomy10091321.
- 78.
Singh, J.S.; Kumar, A.; Rai, A.N.; et al. Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. https://doi.org/10.3389/fmicb.2016.00529.
- 79.
Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; et al. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. https://doi.org/10.1080/07388551.2019.1654972.
- 80.
Parmar, P.; Kumar, R.; Neha, Y.; et al. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy-based solutions. Front. Plant Sci. 2023, 14, 1073546. https://doi.org/10.3389/fpls.2023.1073546.
- 81.
Aloo, B.N.; Tripathi, V.; Makumba, B.A.; et al. Plant growth-promoting rhizobacterial biofertilizers for crop production: The past, present, and future. Front. Plant Sci. 2022, 13, 1002448. https://doi.org/10.3389/fpls.2022.1002448.
- 82.
Suchithra, M.R.; Muniswami, D.M.; Sri, M.S.; et al. Effectiveness of green microalgae as biostimulants and biofertilizer through foliar spray and soil drench method for tomato cultivation. S. Afr. J. Bot. 2022, 146, 740–750. https://doi.org/10.1016/j.sajb.2021.12.022.
- 83.
Refaay, D.A.; El-Marzoki, E.M.; Abdel-Hamid, M.I.; et al. Effect of foliar application with Chlorella vulgaris, Tetradesmus dimorphus, and Arthrospira platensis as biostimulants for common bean. J. Appl. Phycol. 2021, 33, 3807–3815. https://doi.org/10.1007/s10811-021-02584-z.
- 84.
La Bella, E.; Baglieri, A.; Rovetto, E.I.; et al. Foliar spray application of Chlorella vulgaris extract: Effect on the growth of lettuce seedlings. Agronomy 2021, 11, 308. https://doi.org/10.3390/agronomy11020308.
- 85.
Dineshkumar, R.; Duraimurugan, M.; Sharmiladevi, N.; et al. Microalgal liquid biofertilizer and biostimulant effect on green gram (Vigna radiata L.) an experimental cultivation. Biomass Conv. Bioref. 2022, 12, 3007–3027. https://doi.org/10.1007/s13399-020-00857-0.
- 86.
Arahou, F.; Lijassi, I.; Wahby, A.; et al. Spirulina-based biostimulants for sustainable agriculture: Yield improvement and market trends. BioEnergy Res. 2023, 16, 1401–1416. https://doi.org/10.1007/s12155-022-10537-8.
- 87.
Sutherland, D.L.; Ralph, P.J. Microalgal Bioremediation of Emerging Contaminants—Opportunities and Challenges. Water Res. 2019, 164, 114921. https://doi.org/10.1016/j.watres.2019.114921.
- 88.
Mustafa, S.; Bhatti, N.; Maqbool, M. Microalgae Biosorption, Bioaccumulation and Biodegradation Efficiency for the Remediation of Wastewater and Carbon Dioxide Mitigation: Prospects, Challenges and Opportunities. J. Water Process Eng. 2021, 41, 2214–7144. https://doi.org/10.1016/j.jwpe.2021.102009.
- 89.
Hussein, M.H.; Abdullah, A.M.; Badr El Din, N.I.; et al. Biosorption Potential of the Microchlorophyte Chlorella vulgaris for Some Pesticides. J. Fertil. Pestic. 2017, 8, 1000177. https://doi.org/10.4172/2471-2728.1000177.
- 90.
Komárek, M.; Čadková, E.; Chrastný, V.; et al. Contamination of Vineyard Soils with Fungicides: A Review of Environmental and Toxicological Aspects. Environ. Int. 2010, 36, 138–151. https://doi.org/10.1016/j.envint.2009.10.005.
- 91.
Verasoundarapandian, G.; Lim, Z.S.; Radziff, S.B.M.; et al. Remediation of pesticides by microalgae as feasible approach in agriculture: Bibliometric strategies. Agronomy 2022, 12, 117. https://doi.org/10.3390/agronomy12010117.
- 92.
Sarker, N.K.; Kaparaju, P. Microalgal bioeconomy: A green economy approach towards achieving sustainable development goals. Sustainability 2024, 16, 11218. https://doi.org/10.3390/su162411218.
- 93.
Allen, M.; Stainier, R.Y. Growth and Division of Some Unicellular Blue-green Algae. J. Gen. Microbiol. 1968, 51, 199–202. https://doi.org/10.1099/00221287-51-2-199.
- 94.
Fanesi, A.; Raven, J.A.; Giordano, M. Growth rate affects the responses of the green alga Tetraselmis suecica to external perturbations. Plant Cell Environ. 2014, 37, 512–519. https://doi.org/10.1111/pce.12176.
- 95.
Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; et al. Bacteriophages as Weapons Against Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 825. https://doi.org/10.3389/fmicb.2016.00825.
- 96.
Templeton, D.W.; Laurens, L.M.L. Nitrogen-to-protein conversion factors revisited for applications of microalgal biomass conversion to food, feed and fuel. Algal Res. 2015, 11, 359–367. https://doi.org/10.1016/j.algal.2015.07.013.
- 97.
Rizzo, M.G.; Morganti, D.; Smeriglio, A.; et al. Formation of 3D Human Osteoblast Spheroids Incorporating Extracellular Matrix-Mimetic Phage Peptides as a Surrogate Bone Tissue Model. Int. J. Mol. Sci. 2025, 26, 8482. https://doi.org/10.3390/ijms26178482.
- 98.
Rizzo, M.G.; Fazio, E.; De Pasquale, C.; et al. Physiopathological Features in a Three-Dimensional In Vitro Model of Hepatocellular Carcinoma: Hypoxia-Driven Oxidative Stress and ECM Remodeling Cancers 2025, 17, 3082. https://doi.org/10.3390/cancers17183082.
- 99.
Rizzo, M.G.; Cordaro, M.; Morganti, D.; et al. Osteogenic Activity and Bone Matrix Mineralization Induced by Vitis vinifera Leaves Extract in Human Osteoblastic Cells. Food Sci. Nutr. 2025, 13, e70785. https://doi.org/10.1002/fsn3.70785.
- 100.
Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; et al. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. https://doi.org/10.3390/md14050100.
- 101.
Bossa, R.; Di Colandrea, M.; Salbitani, G.; et al. Phosphorous utilization in microalgae: Physiological aspects and applied implications. Plants 2024, 13, 2127. https://doi.org/10.3390/plants13152127.
- 102.
Deepa, P.; Sowndhararajan, K.; Kim, S. A review of the harvesting techniques of microalgae. Water 2023, 15, 3074. https://doi.org/10.3390/w15173074.
- 103.
Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 12 October 2025).

This work is licensed under a Creative Commons Attribution 4.0 International License.


