- Characterizing degassing, boiling, and mixing in the Trans-Himalayan geothermal fluids.
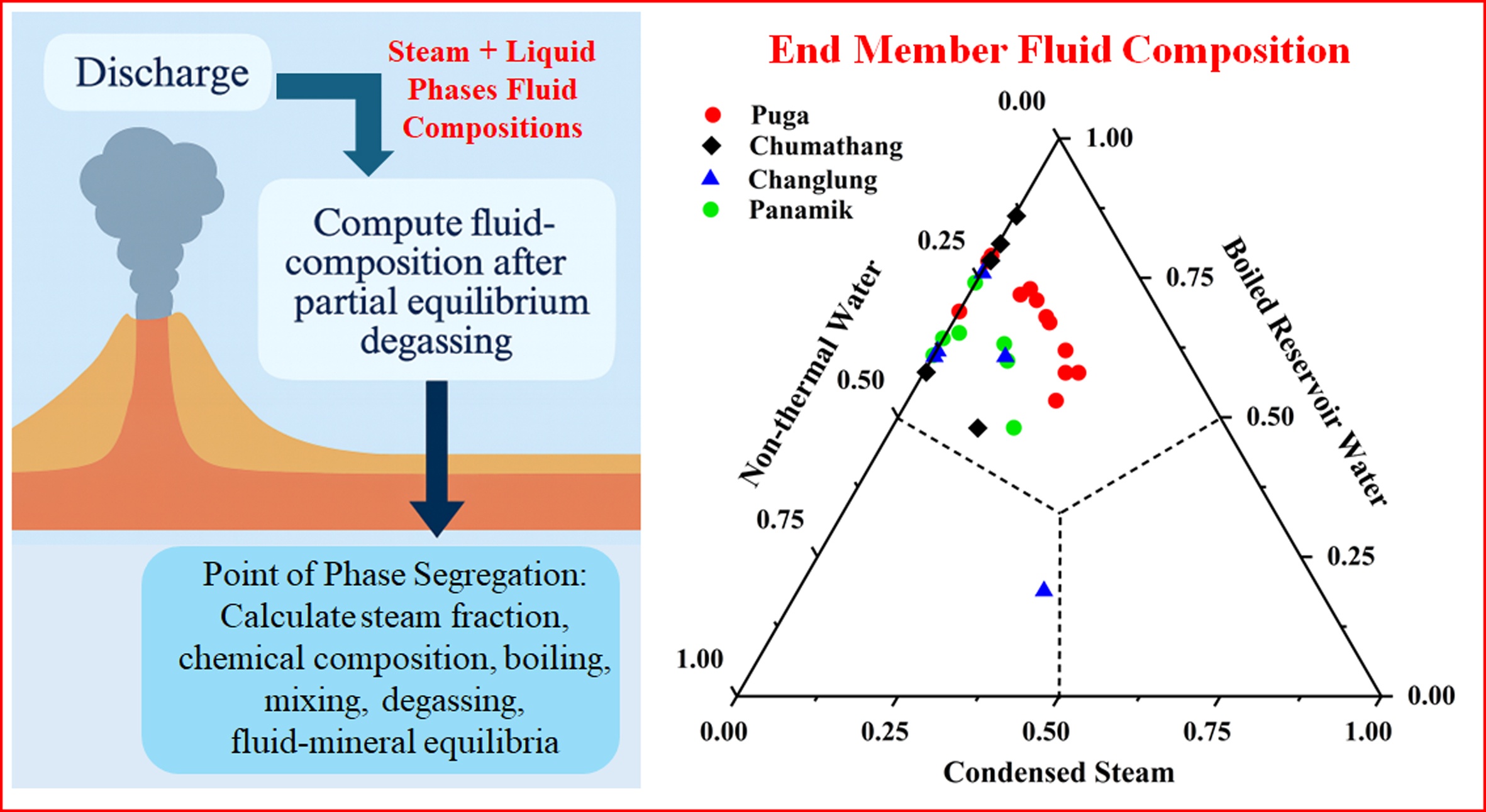
- The surface geothermal water is a mixture of three end-members (non-thermal water, boiled reservoir water, and condensed steam).
- Geochemical modeling shows that all geothermal fluids have >40% of boiled deep fluids.
- Results highlight the role of boiling and mixing that shaped the fluid composition of the Trans-Himalayan geothermal systems.
- Open Access
- Article
Boiling, Degassing, and Mixing of Fluids in the Trans-Himalayan Geothermal Systems, India
- Archisman Dutta 1, 2, *,
- Sitangshu Chatterjee 3,
- Parashar Mishra 4,
- Ashok Singh 1,
- Anubha Bhandari 1, *,
- Muduru Lachhana Dora 5,
- Pramod Kumar Singh 6,
- Biswajit Ray 2,
- Vivek Prakash Malviya 7
Author Information
Received: 15 Dec 2025 | Revised: 15 Jan 2026 | Accepted: 22 Jan 2026 | Published: 29 Jan 2026
Highlights
Abstract
Geothermal fluids, during ascent, are subjected to various secondary processes which alter their chemistry from reservoir to surface discharge. We have characterized and quantified various secondary processes such as degassing, boiling, and mixing in this study for the Trans-Himalayan geothermal fluids. The geochemical facies of thermal waters are found to be Na–Cl for Puga, mixed type (Na–Cl–SO4–HCO3 ) for Chumathang and Panamik, and Na–HCO3 type for Changlung having neutral to moderately alkaline pH (6.7–8.9) along with TDS ranging from 525 mg/l to 2931 mg/l. The geothermal reservoir temperature cal- culated from the Na–K cation geothermometry varies between 170–260◦C. The reser- voir fluids, constructed by adding liquid and steam phases compositions, exhibit near- neutral pH (6.47–7.03) with lower TDS than surface discharges (∼561–1811 mg/l). The surface geothermal water is found to be the resultant mixture of three end-members (non- thermal water, boiled reservoir water, and condensed steam) based on which a ternary mixing model has been developed. The SiO2–temperature relation indicates undersatu- ration with respect to amorphous silica but equilibrium to supersaturation with respect to chalcedony and quartz. This pattern implies boiling-driven fluid evolution, kinetically con- trolled silica scaling, and progressively more mature reservoir waters from Panamik and Puga geothermal area. Geochemical modeling result shows that all geothermal fluids have >40% of boiled deep fluids, traced with conservative component, Cl. The fraction of con- densed steam is highest in Changlung (∼ 11–34%) and lowest in Chumathang (∼ 13%) and Panamik (∼2–19%) with Puga lying in the intermediate stage (∼8–24%) (traced with reactive component, dissolved CO2 ). All thermal waters exhibit mixing with local meteoric waters, with cold-water contributions ranging from 14–42%. Overall, these results highlight the role of boiling and mixing phenomenon shaping the fluid composition of the Trans- Himalayan geothermal systems.
Graphical Abstract
Keywords
decompression boiling | secondary processes | deep fluid reconstruction | end-members fluid compositions
References
- 1.
Giggenbach, W.F. Geothermal solute equilibria. Derivation of Na-KMg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749– 2765.
- 2.
Arnrósson, S.; Stefánsson, A.; Bjarnason, J.O. Fluid–fluid interactions in geothermal systems. Rev. Mineral. Geochem. 2007, 65, 259–312.
- 3.
Balaram, V.; Santosh, M. Critical metal deposits in terrestrial and oceanic environments and the global energy transition. Habitable Planet 2025, 1, 86–107.
- 4.
Nuñez-Hernández, S.; Pinti, D.L.; López-Hernández, A.; et al. Phase segregation, boiling, and reinjection at the Los Azufres Geothermal Field, Mexico, monitored by water stable isotopes, chloride, and enthalpy. J. Volcanol. Geotherm. Res. 2020, 390, 106751.
- 5.
Gunnarsson-Robin, J.; Stefánsson, A.; Ono, S.; et al. Sulfur isotopes in Icelandic thermal fluids. J. Volcanol. Geotherm. Res. 2017, 346, 161–179.
- 6.
Heřmanská, M.; Stefánsson, A.; Scott, S. Supercritical fluids around magmatic intrusions: IDDP-1 at Krafla, Iceland. Geothermics 2019, 78, 101–110.
- 7.
Dutta, A.; Mishra, P.; Mukherjee, A.; et al. Geochemical evolution of geothermal waters in Trans-Himalayas: Implications for critical mineral deposition. Geochemistry 2025, 85, 126348.
- 8.
Ricci, A.; Kleine, B.I.; Fiebig, J.; et al. Equilibrium and kinetic controls on molecular hydrogen abundance and hydrogen isotope fractionation in hydrothermal fluids. Earth Planet. Sci. Lett. 2022, 579, 117338.
- 9.
Chatterjee, S.; Sharma, S.; Ansari, M.A.; et al. Characterization of subsurface processes estimation of reservoir temperature in TuralRajwadi geothermal fields, Maharashtra, India. Geothermics 2016, 59, 77–89.
- 10.
Chatterjee, S.; Mishra, P.; Keesari, T.; et al. Why is it imperative to use multicomponent geothermometry in medium/low enthalpy thermal waters? Insights from the Gujarat geothermal region, India. Environ. Earth Sci. 2023, 82, 557.
- 11.
Chandrasekharam, D.; Bundschuh, J. Low-Enthalpy Geothermal Resources for Power Generation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007.
- 12.
Sakhare, V.V.; Biswal, B.P.; Rajan, L.C.; et al. Geothermal Atlas of India; GSI Special Publ. 125; Geological Survey of India: Kolkata, India, 2022.
- 13.
Mishra, P.; Absar, A.; Dutta, A.; et al. Hot springs of Demchok, Ladakh, India. Curr. Sci. 2023, 124, 1104–1107.
- 14.
Craig, J.; Absar, A.; Bhat, G.; et al. Hot springs and the geothermal energy potential of Jammu & Kashmir State, NW Himalaya, India. Earth Sci. Rev. 2013, 126, 156–177.
- 15.
Tiwari, S.K.; Rai, S.K.; Bartarya, S.K.; et al. Stable isotopes (δ13 C DIC , δD, δ18 O) and geochemical characteristics of geothermal springs of Ladakh and Himachal (India): Evidence for CO2 discharge in northwest Himalaya. Geothermics 2016, 64, 314–330.
- 16.
Dutta, A.; Mishra, P.; Absar, A.; et al. Tracing hydrothermal mineral thenardite in geysers/hot springs of North-western Himalayan belt, Ladakh Geothermal Province, India by hydrogeochemistry, fluidmineral equilibria and isotopic studies. Geochemistry 2023, 83, 125973.
- 17.
Clark, D.E.; Galeczka, I.M.; Gslason, S.R.; et al. Does the release of toxic metals due to subsurface CO2 storage in basalts pose an environmental hazard? Int. J. Greenh. Gas Control 2026, 149, 104526.
- 18.
Shanker, R.; Absar, A.; Srivastava, G.C.; et al. A case study of Puga geothermal system, India. In Proceedings of the 22nd NZ Geothermal Workshop, Auckland, New Zealand, 2000. ISBN: 0-86869-0244.
- 19.
Scott, S.; Gunnarsson, I.; Arnórsson, S.; et al. Gas chemistry, boiling and phase segregation in a geothermal system, Hellisheidi, Iceland. Geochim. Cosmochim. Acta 2014, 124, 170–189.
- 20.
Stefánsson, A.; Keller, N.S.; Robin, J.G.; et al. Quantifying mixing, boiling, degassing, oxidation and reactivity of thermal waters at Vonarskard, Iceland. J. Volcanol. Geotherm. Res. 2016, 309, 53–62.
- 21.
Guo, Q.; Wang, Y.; Liu, W. O, H, and Sr isotope evidences of mixing processes in two geothermal fluid reservoirs at Yangbajing, Tibet, China. Environ. Earth Sci. 2010, 59, 1589–1597.
- 22.
Zhang, Y.; Xiao, Y.; Yang, H.; et al. Hydrogeochemical and isotopic insights into the genesis and mixing behaviors of geothermal water in a faults-controlled geothermal field on Tibetan Plateau. J. Clean. Prod. 2024, 442, 140980.
- 23.
Hurwitz, S.; Stefánsson, A.; Shock, E.L.; et al. The geochemistry of continental hydrothermal systems. In Treatise on Geochemistry, 3rd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 301–345.
- 24.
Scott, S.; Driesner, T.; Weis, P. Boiling and condensation of saline geothermal fluids above magmatic intrusions. Geophys. Res. Lett. 2017, 44, 1696–1705.
- 25.
Searle, M.; Cooper, D.; Rex, A.; et al. Collision tectonics of the Ladakh-Zanskar Himalaya. Philos. Trans. R. Soc. 1988, 326, 117– 150.
- 26.
Thakur, V.C.; Misra, D.K. Tectonic framework of the Indus and Shyok suture zones in Eastern Ladakh, Northwest Himalaya. Tectonophysics 1984, 110, 77–93.
- 27.
de Sigoyer, J.; Chavagnac, V.; Blichert-Toft, J.; et al. Dating the Indian continental subduction and collisional thickening in the northwest Himalaya: Multichronology of the Tso Morari eclogites. Geology 2000, 28, 487–490.
- 28.
Guillot, S.; de Sigoyer J.; Lardeaux, J.; et al. Eclogitic metasediments from the Tso Morari area (Ladakh, Himalaya): Evidence for continental subduction during India-Asia convergence. Contrib. Mineral Petrol. 1997, 128, 197–212.
- 29.
Bhargava, O.N. The Geology of the Indus Suture Zone in Ladakh, Himalaya; Geological Survey of India: Calcutta, India, 1995.
- 30.
Honegger, K.; Dietrich, V.; Frank, W.; et al. Magmatism and metamorphism in the Ladakh Himalayas (the Indus-Tsangpo suture zone). Earth Planet. Sci. Lett. 1982, 60, 253–292.
- 31.
Pedersen, R.B.; Searle, M.T.; Corfield, R.I. U–Pb zircon ages from the Spontang ophiolite, Ladakh Himalaya. J. Geol. Soc. 2001, 158, 513–520.
- 32.
Reuber, I. The Dras arc: Two successive volcanic events on eroded oceanic crust. Tectonophysics 1989, 161, 93–106.
- 33.
Mahéo, G.; Bertrand, H.; Guillot, S.; et al. The South Ladakh ophiolites (NW Himalaya, India): An intra-oceanic tholeiitic arc origin with implication for the closure of the Neo-Tethys. Chem. Geol. 2004, 203, 273–303.
- 34.
Garzanti, E.; Van Haver, T. The Indus Clastics: Forearc basin sedimentation in the Ladakh Himalaya. Sediment. Geol. 1988, 59, 237– 249.
- 35.
Clift, P.D.; Carter, A.; Krol, M.; et al. Constraints of India–Eurasia collision in the Arabian Sea region taken from the Indus Group, Ladakh Himalaya, India. In The Tectonic and Climatic Evolution of the Arabian Sea Region; Clift, P.D., Kroon, D., Gaedicke, C., et al., Eds.; Geol. Soc. Spec. Pub. 195; Geological Society of London: London, UK, 2002; pp. 97–116.
- 36.
Searle, M.; Weinberg, R.F.; Dunlap, W.J. Transpressional tectonics along the Karakoram fault zone, northern Ladakh: Constraints on Tibetan extrusion. Geol. Soc. Spec. Publ. 1998, 135, 307–326.
- 37.
Ravikant, V.; Wu, F.Y.; Ji, W.Q.; Zircon U-Pb and Hf isotopic constraints on petrogenesis of the Cretaceous-Tertiary granites in eastern Karakoram and Ladakh, India. Lithos 2009, 110, 153–166.
- 38.
Debon, F.; Ali Khan, N. Alkaline orogenic plutonism in the Karakorum batholith: The Upper Cretaceous Koz Sar complex (Karambar valley, N. Pakistan). Geodin. Acta 1996, 9, 145–160.
- 39.
Zhang, M.; Xie, X.-G.; Liu, W.; et al. Hydrothermal degassing through the Karakoram fault, western Tibet: Insights into active deformation driven by continental strike-slip faulting. Geophys. Res. Lett. 2024, 51, e2023GL106647.
- 40.
Geological Map of India. Geological Survey of India, 1998. Available online: www.gsi.gov.in (accessed on 1 December 2025).
- 41.
Mishra, P.; Dutta, A.; Absar, A.; et al. Tracing the evolution of shallow geothermal springs in the Shyok–Nubra Valley of North-West Himalayas, India through hydrogeochemistry and stable isotopes (δ18 O, δD). Solid Earth Sci. 2024, 9, 100175.
- 42.
Arnórsson, S.; Bjarnason, J.Ö.; Giroud, N.; et al. Sampling and analysis of geothermal fluids. Geofluids 2006, 6, 203–216.
- 43.
APHA (American Public Health Association, American Water Works Association, and Water Environment Federation). Standard Methods for the Examination of Water and Wastewater, 24th ed.; American Public Health Association (APHA): Washington DC, USA, 2023.
- 44.
Ármannsson, H.; Óskarsson, F.; Fridriksson, T. Geochemical exploration techniques. In Comprehensive Renewable Energy, 2nd ed.; Elsevier: Oxford, UK, 2022; pp. 80–97.
- 45.
Arnórsson, S. Isotopic and Chemical Techniques in Geothermal Exploration, Development, and Use ; International Atomic Energy Agency (IAEA): Vienna, Austria, 2000; pp. 66–248.
- 46.
Bjarnason, J.Ö. The Chemical Speciation Program WATCH, version 2.4; SOR Iceland: Reykjavk, Iceland, 2010.
- 47.
Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations (No. 99-4259), Version 2; US Geological Survey: Reston, VA, USA, 1999.
- 48.
Giggenbach, W.F. Graphical techniques for the evaluation of water/rock equilibration conditions by use of Na, K, Mg and Ca contents of discharge waters. In Proceedings of the 8th New Zealand Geothermal Workshop, Auckland, New Zealand, 21–23 January 1986, pp. 37–44.
- 49.
Tiwari, S.K.; Yadav, J.S.; Sain, K.; et al. Impact of geothermal activity on the anomalous retreat of Changmolung Glacier in the Karakoram. Sci. Tot. Environ. 2025, 999, 180332.
- 50.
Jeelani, G.; Lone, S. A.; Deshpande, R. D.; et al. Origin and evolution of fluids and heatflow in geothermal systems of Indus River Basin (IRB), India. Sci. Rep. 2025, 15, 43746.
- 51.
Kumar, P.V.; Patro, P.K.; Azeez, K.A.; et al. Inferred secondary magma pathways between Puga and Chumathang geothermal systems from magnetotelluric data in the Himalayan collisional zone. Earth Planets Space 2026, 78, 3.
- 52.
Nicholson, K. Geothermal Fluids Chemistry and Exploration Techniques, 1st ed.; Springer-Verlag: Berlin, Heidelberg, Germany, 1993.
- 53.
Dutta, A.; Mishra, P.; Thapliyal, A.P.; et al. Implication of epithermal mineralization as proxy for geothermal energy potentiality in Puga, Ladakh UT, India. Earth Sci. 2024, 13, 8–13.
- 54.
Ansari, A.H.; Das, A.; Ansari, N.G.; et al. Tracing early life on Mars: Lessons from organics produced in high-altitude hot springs of Ladakh. Prog. Earth Planet. Sci. 2025, 12, 1–19.
- 55.
Sarkar, S.; Moitra, H.; Bhattacharya, S.; et al. 2022. Spectroscopic studies on the Puga Hot Spring Deposits, Ladakh, an astrobiological Martian analog site in India. J. Geophys. Res. Planets 2022, 127, e2022JE007299.
- 56.
Phartiyal, B.; Kumar, A.; Shukla, S. Martian/Lunar analogue research station in India: Ladakh as a potential site. Curr. Sci. 2025, 128, 00113891.
- 57.
Chaddha, A.S.; Shukla, S.K.; Sharma, A.; et al. Calcium carbonate as a potential template for the origin of life: Coupled inorganic– organic geochemistry of travertine deposit from Puga hot spring. ACS Earth Space Chem. 2025, 9, 1905–1926.
- 58.
Mishra, P.; Dutta, A.; Malviya, V.P.; et al. Conceptual modelling on water-rock reaction and genesis of high pH fluids in a typical granitoid geothermal reservoir: A case from Indus-Tsangpo Suture Zone, India. Phys. Chem. Earth Parts A/B/C 2024, 136, 103736.
- 59.
Axelsson, G.; Arnaldsson, A.; Ármannsson, H.; et al. Updated conceptual model and capacity estimates for the Greater Olkaria geothermal system, Kenya. In Proceedings of the Thirty-Eighth Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 11–13 February 2013.
- 60.
Ciriaco, A.E.; Zarrouk, S.J.; Zakeri, G. Geothermal resource and reserve assessment methodology: Overview, analysis and future directions. Renew. Sustain. Energy Rev. 2020, 119, 109515.
- 61.
Stefánsson, A.; Barnes, J.D. Chlorine isotope geochemistry of Icelandic thermal fluids: Implications for geothermal system behavior at divergent plate boundaries. Earth Planet. Sci. Lett. 2016, 449, 69–78.
- 62.
Arnórsson, S.; Angcoy, E.; Bjarnason, J.Ö.; et al. Gas chemistry of volcanic geothermal systems. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–30 April 2010.
- 63.
Gunnarsson, I.; Arnórsson, S. Amorphous silica solubility and the thermodynamic properties of H4SiO4 in the range of 0 to 350◦C at Psat . Geochim. Cosmochim. Acta 2000, 64, 2295–2307.
- 64.
Stefánsson, A.; Arnórsson, S. Feldspar saturation state in natural waters. Geochim. Cosmochim. Acta 2000, 64, 2567–2584.
- 65.
Arnrsson, S.; Andrésdóttir, A. Processes controlling the distribution of boron and chlorine in natural waters in Iceland. Geochim. Cosmochim. Acta 1995, 59, 4125–4146.
- 66.
Tole, M.P.; Ármannsson, H.; Zhong-He, P.; et al. Fluid/mineral equi-librium calculations for geothermal fluids and chemical geothermometry. Geothermics 1993, 22, 17–37.
- 67.
Lelli, M.; Kretzschmar, T.G.; Cabassi, J.; et al. Fluid geochemistry of the Los Humeros geothermal field (LHGF-Puebla, Mexico): New constraints for the conceptual model. Geothermics 2021, 90, 101983.
- 68.
Patro, P.K.; Dhamodharan, S.; Durga, V.; et al. Delineation of a geothermal source beneath the Panamik-Changlung Hot Springs along the Karakoram Fault, Ladakh, India, using magnetotelluric studies. J. Volcanol. Geotherm. Res. 2025, 468, 108478.
- 69.
Kumar, D.; Ojha, A.K.; Maurya, V.P.; et al. New insight into the shallow sub-surface for geothermal prospection of Puga valley, Ladakh, India from electrical resistivity tomography. Geothermics 2025, 131, 103409.

This work is licensed under a Creative Commons Attribution 4.0 International License.


