- Global cesium supply remains narrow, relying on few pegmatite suites and facing rising strategic demand.
- High-temperature geothermal systems in the Tibetan Plateau host unusually cesium-rich waters and siliceous deposits.
- Multiple lines of evidence support a deep crustal origin, with rare-metal melts feeding upward-moving cesiumbearing fluids.
- Tibetan active springs and shallow siliceous deposits together form substantial cesium resources.
- Shallow geothermal cesium is best developed through distributed co-production with heat and power generation.
- Open Access
- Review
Geothermal Cesium Resources in the Tibetan Plateau: Geological Controls, Resource Potential, and Implications for Low-Carbon Energy Transition
Author Information
Received: 30 Nov 2025 | Revised: 26 Dec 2025 | Accepted: 04 Jan 2026 | Published: 13 Jan 2026
Highlights
Abstract
Cesium (Cs) is a strategic metal used in high-precision timing and advanced electronics technologies, but current supply comes mainly from a few pegmatites as associated minerals. This concentration, together with rising demand, creates clear risks for global supply chains. In this context, this study reviews the geological setting, enrichment processes and resource potential of unique geothermal-type Cs resources on the Tibetan Plateau, and its relevance for critical metal security and the energy transition. Hydrochemical, isotopic, petrological and geophysical data show that southern Tibet hosts a distinct geothermal Cs province, where high-temperature systems along Yarlung Zangbo Suture and N–S trending rifts are consistently enriched in Cs in both fluids and siliceous deposits, well above levels in most other geothermal fields worldwide. The evidence supports a crustal evolved magmatic–hydrothermal fluid source model: Himalayan crust undergoes partial melting and magmatic differentiation, releases Cs-rich fluids that rise along fault zones, and the mixed geothermal waters are then trapped in opal-rich siliceous sinters, ancient siliceous rocks and sediment-hosted units. Tibetan geothermal systems therefore contain a dual Cs resource, with both a dissolved flux and a shallow solid inventory in siliceous sinters and sedimentary rocks. Geothermal Cs on the Tibetan Plateau represents a separate geothermal-type deposit, marked by high enrichment, shallow occurrence and close coupling to geothermal heat. Its dispersed, small- to medium-scale nature makes it best suited to co-production with geothermal development. It can enhance the diversity and resilience of Cs supply, while supporting integrated strategies for low-carbon energy deployment and critical metal security.
Graphical Abstract
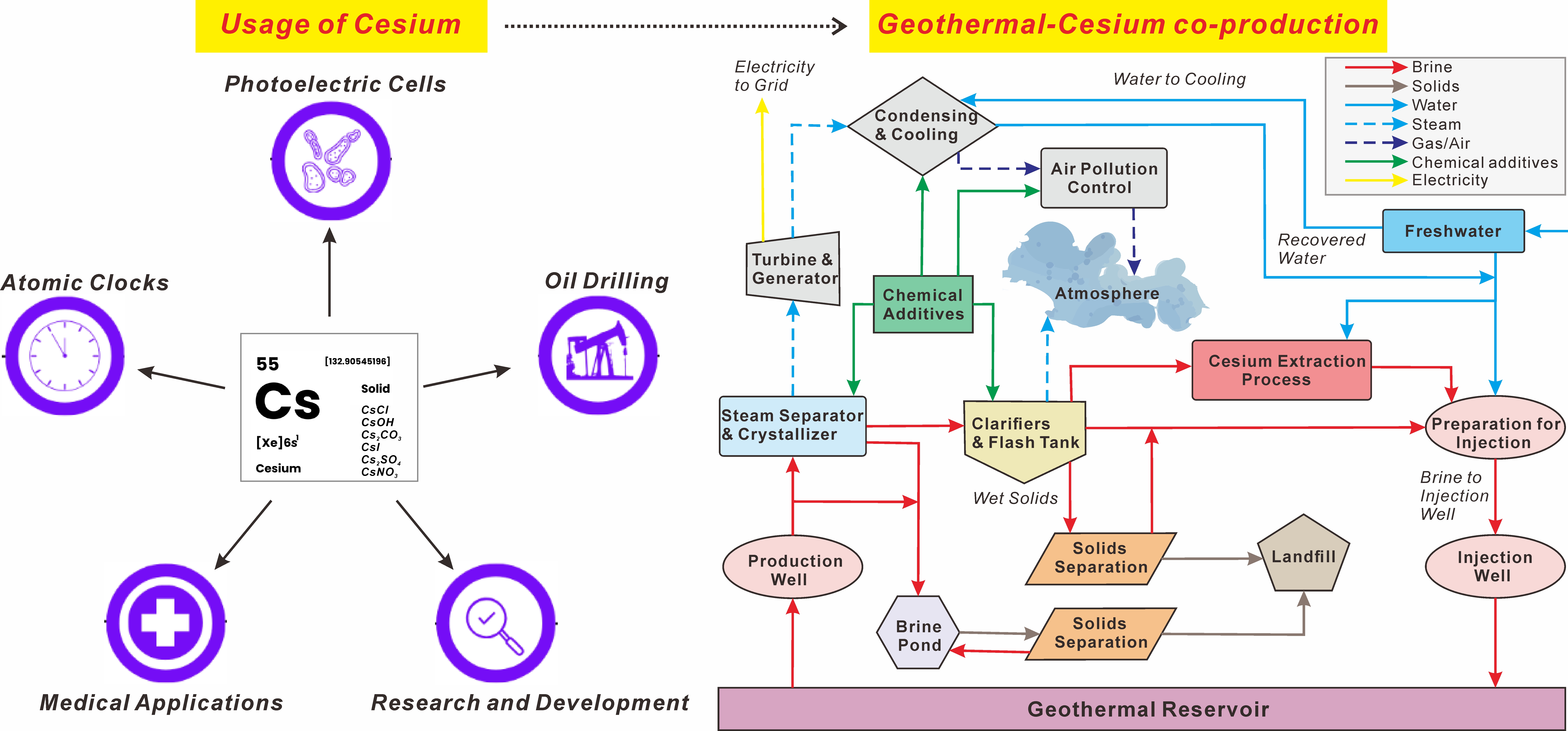
Keywords
Cesium mineralization | Geothermal systems | Critical mineral resources | Geothermal–metal co-production | Tibetan Plateau
References
- 1.
Santosh, M.; Groves, D.I.; Yang, C.-X. Habitable planet to sustainable civilization: Global climate change with related clean energy transition reliant on declining critical metal resources. Gondwana Res. 2024, 130, 220–233. https://doi.org/10.1016/j.gr.2024.01.013
- 2.
Shang, Y.; Sang, S.; Tiwari, A.K.; et al. Impacts of renewable energy on climate risk: A global perspective for energy transition in a climate adaptation framework. Appl. Energy 2024, 362, 122994. https://doi.org/10.1016/j.apenergy.2024.122994
- 3.
Gielen, D. Critical Minerals for the Energy Transition; International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2021.
- 4.
Mathieux, F.; Ardente, F.; Bobba, S.; et al. Critical Raw Materials and the Circular Economy; Publications Office of the European Union: Bruxelles, Belgium, 2017.
- 5.
Gulley, A.L.; Nassar, N.T.; Xun, S.; et al. China, the United States, and competition for resources that enable emerging technologies. Proc. Natl. Acad. Sci. USA 2018, 115, 4111–4115. https://doi.org/10.1073/pnas.1717152115
- 6.
Muller, D.; Groves, D.I.; Santosh, M.; et al. Critical metals: Their applications with emphasis on the clean energy transition. Geosyst. Geoenviron. 2025, 4, 100310. https://doi.org/10.1016/j.geogeo.2024.100310
- 7.
Varala, R.; Rao, K.S. Cesium salts in organic synthesis: A review. Curr. Org. Chem. 2015, 19, 1242–274. https://doi.org/10.2174/1385272819666150507220755
- 8.
Gao, X.R.; Jia, H.X.; Li, T.J.; et al. Perspective of rubidium and caesium resource demand in China. Acta Geosci. Sin. 2023, 44, 279–285. https://doi.org/10.1111/1755-6724.14302
- 9.
Stilling, A.; C´ erny´ , P.; Vanstone, P.J. The Tanco Pegmatite at Bernic Lake, Manitoba. XVI. Zonal and bulk compositions and their petrogenetic significance. Can. Mineral. 2006, 44, 599–623. https://doi.org/10.2113/gscanmin.44.3.599
- 10.
Bradley, D.; McCauley, A.D.; Stillings, L.L. Mineral-Deposit Model for Lithium-Cesium-Tantalum Pegmatites; U.S. Geological Survey: Reston, VA, USA, 2017. https://doi.org/10.3133/sir20105070O
- 11.
U.S. Geological Survey. Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025. https://doi.org/10.3133/mcs2025
- 12.
Mudd, G.M.; Jowitt, S.M. Global resource assessments of primary metals: An optimistic reality check. Nat. Resour. Res. 2018, 27, 229–240. https://doi.org/10.1007/s11053-017-9349-0
- 13.
Alms, K.; Jagert, F.; Blomer, J.; et al. Co-production of geothermal energy and lithium from geothermal waters. In Proceedings of the European Congress, Berlin, Germany, 17–21 October 2022; European Geothermal Energy Council (EGEC): Brussels, Belgium, 2022.
- 14.
Goldberg, V.; Dashti, A.; Egert, R.; et al. Challenges and opportunities for lithium extraction from geothermal systems in Germany—Part 3: The return of the extraction brine. Energies 2023, 16, 5899. https://doi.org/10.3390/en16165899
- 15.
Koelbel, L.; Kolbel, T.; Herrmann, L.; et al. Lithium extraction from geothermal brines in the Upper Rhine Graben: A case study of potential and current state of the art. Hydrometallurgy 2023, 221, 106131. https://doi.org/10.1016/j.hydromet.2023.106131
- 16.
Sanjuan, B.; Gourcerol, B.; Millot, R.; et al. Lithium-rich geothermal brines in Europe: An up-date about geochemical characteristics and implications for potential Li resources. Geothermics 2022, 101, 102385. https://doi.org/10.1016/j.geothermics.2022.102385
- 17.
Schenker, V.; Oberschelp, C.; Pfister, S. Regionalized life cycle assessment of present and future lithium production for Li-ion batteries. Resour. Conserv. Recycl. 2022, 187, 106611. https://doi.org/10.1016/j.resconrec.2022.106611
- 18.
Weinand, J.M.; Vandenberg, G.; Risch, S.; et al. Low-carbon lithium extraction makes deep geothermal plants cost-competitive in future energy systems. Adv. Appl. Energy 2023, 11, 100148. https://doi.org/10.1016/j.adapen.2023.100148
- 19.
Busse, M.M.; McKibben, M.A.; Stringfellow, W.; et al. Impact of geothermal expansion and lithium extraction in the Salton Sea known geothermal resource area (SS-KGRA) on local water resources. Environ. Res. Lett. 2024, 19, 104011. https://doi.org/10.1088/1748-9326/ad6a73
- 20.
Subasinghe, H.C.S.; Zhang, H.; Shi, F.; et al. Critical minerals extraction from geothermal brines. Joule 2025, 9, 102171. https://doi.org/10.1016/j.joule.2025.102171
- 21.
Wang, D.; Xue, F.; Ren, L.; et al. Critical minerals in Tibetan geothermal systems: Their distribution, flux, reserves, and resource effects. Minerals 2025, 15, 93. https://doi.org/10.3390/min15010093
- 22.
Banshoya, S.I.; Berre, I.; Keilegavlen, E. A simulation study of the impact of fracture networks on the co-production of geothermal energy and lithium. Geotherm. Energy 2025, 13, 31. https://doi.org/10.1186/s40517-025-00356-3
- 23.
Grimaud, D.; Huang, S.; Michard, G.; et al. Chemical study of geothermal waters of Central Tibet (China). Geothermics 1985, 14, 35–48. https://doi.org/10.1016/0375-6505(85)90092-6
- 24.
Tan, H.B.; Shi, Z.W.; Cong, P.X.; et al. The spatial distribution law of B, Li, Rb and Cs elements and supernormal enrichment mechanism in Tibet geothermal system. Sediment. Geol. Tethyan Geol. 2023, 43, 404–415. https://doi.org/10.19826/j.cnki.1009-3850.2023.02001
- 25.
Xue, F.; Tan, H.; Zhang, X.; et al. Sources, enrichment mechanisms, and resource effects of rare metal elements-enriched geothermal springs in Xizang, China. Sci. China Earth Sci. 2024, 67, 3476–3499. https://doi.org/10.1007/s11430-024-1413-0
- 26.
Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Oxford, UK, 2012.
- 27.
Sun, Y.; Wang, D.H.; Wang, C.H.; et al. Metallogenic regularity, new prospecting and guide direction of rubidium deposits in China. Acta Geol. Sin. 2019, 93, 1231–1244. https://doi.org/10.19762/j.cnki.dizhixuebao.2019183
- 28.
Klemperer, S.L.; Zhao, P.; Whyte, C.J.; et al. Limited underthrusting of India below Tibet: 3He/4He analysis of thermal springs locates the mantle suture in continental collision. Proc. Natl. Acad. Sci. USA 2022, 119, e2113877119. https://doi.org/10.1073/pnas.2113877119
- 29.
Tamburello, G.; Chiodini, G.; Ciotoli, G.; et al. Global thermal spring distribution and relationship to endogenous and exogenous factors. Nat. Commun. 2022, 13, 6378. https://doi.org/10.1038/s41467-022-34115-w
- 30.
Elenga, H.I.; Tan, H.; Su, J.; et al. Origin of the enrichment of B and alkali metal elements in the geothermal water in the Tibetan Plateau: Evidence from B and Sr isotopes. Geochemistry 2021, 81, 125797. https://doi.org/10.1016/j.chemer.2021.125797
- 31.
Guo, Q.; Nordstrom, D.K.; McCleskey, R.B. Towards understanding the puzzling lack of acid geothermal springs in Tibet (China): Insight from a comparison with Yellowstone (USA) and some active volcanic hydrothermal systems. J. Volcanol. Geotherm. Res. 2014, 288, 94–104. https://doi.org/10.1016/j.jvolgeores.2014.10.005
- 32.
Tan, H.; Su, J.; Xu, P.; et al. Enrichment mechanism of Li, B and K in the geothermal water and associated deposits from the Kawu area of the Tibetan Plateau: Constraints from geochemical experimental data. Appl. Geochem. 2018, 93, 60–68. https://doi.org/10.1016/j.apgeochem.2018.04.001
- 33.
Wang, C.; Zheng, M.; Zhang, X.; et al. Geothermal-type lithium resources in southern Xizang, China. Acta Geol. Sin. 2021, 95, 860–872. https://doi.org/10.1111/1755-6724.14675
- 34.
Li, J.; Wang, X.; Ruan, C.; et al. Enrichment mechanisms of lithium for the geothermal springs in southern Tibet, China. J. Hydrol. 2022, 612, 128022. https://doi.org/10.1016/j.jhydrol.2022.128022
- 35.
Li, Y.-C.; Wei, H.-Z.; Palmer, M.R.; et al. Boron isotope constraints on the migration and accumulation of rare alkali metals in the geyserite cesium deposits in southern Tibet. Ore Geol. Rev. 2023, 163, 105740. https://doi.org/10.1016/j.oregeorev.2023.105740
- 36.
Li, J.; Li, H.; Ruan, C.; et al. Hydrochemical insights into lithium enrichment mechanisms in southern Tibet’s geothermal systems. J. Geochem. Explor. 2026, 280, 107929. https://doi.org/10.1016/j.gexplo.2025.107929
- 37.
Li, Y.-L.; Miao,W.-L.; He, M.-Y.; et al. Origin of lithium-rich salt lakes on the western Kunlun Mountains of the Tibetan Plateau: Evidence from hydrogeochemistry and lithium isotopes. Ore Geol. Rev. 2023, 155, 105356. https://doi.org/10.1016/j.oregeorev.2023.105356
- 38.
Miao, W.; Zhang, X.; Li, Y.; et al. Lithium and strontium isotopic systematics in the Nalenggele River catchment of Qaidam Basin, China: Quantifying contributions to lithium brines and deciphering lithium behavior in hydrological processes. J. Hydrol. 2022, 614, 128630. https://doi.org/10.1016/j.jhydrol.2022.128630
- 39.
Shi, Z.; Tan, H.; Xue, F.; et al. Hydrochemical evolution and source mechanisms governing the unusual lithium and boron enrichment in salt lakes of northern Tibet. Geol. Soc. Am. Bull. 2024, 136, 5174–5190. https://doi.org/10.1130/B37516.1
- 40.
Xue, F.; Tan, H.; Zhang, X.; et al. Contrasting sources and enrichment mechanisms in lithium-rich salt lakes: A Li–H–O isotopic and geochemical study from northern Tibetan Plateau. Geosci. Front. 2024, 15, 101768. https://doi.org/10.1016/j.gsf.2023.101768
- 41.
Xue, F.; Tan, H.; Zhang, X.; et al. Geochemical behavior and migration processes of lithium in the coupled geothermal springriver-salt lake mineralization system in northern Xizang. Acta Petrol. Sin. 2025, 41, 968–982. https://doi.org/10.18654/1000-0569/2025.03.16
- 42.
Zheng, M.; Wang, Q.; Duo, J.; et al. A New Type of Hydrothermal Deposit Cesium-bearing Geyserite in Tibet; Geological Publishing House: Beijing, China, 1995; pp. 113–172.
- 43.
Zhao, Y.; Nie, F.; Hou, Z.; et al. Geological characteristics and formation age of hot spring cesium deposit in Targejia area, Tibet. Miner. Depos. 2006, 25, 281–291. https://doi.org/10.3969/j.issn.1001-3458.2006.03.003
- 44.
Zhao, Y.; Fan, X.; Han, J.; et al. Geologic and geochemical features and ore forming process for hot spring cesium deposit of Gulu area, Nagqu region, Tibet, China. Geol. Bull. China 2009, 28, 933–954. https://doi.org/10.3969/j.issn.1671-2552.2009.07.014
- 45.
Wang, W.; Jiang, S.-Y. The silicon isotopic compositions of silica sinters in Xizang, China: Implications for paleo-geothermal activities since 0.5 Ma B.P. Appl. Geochem. 2024, 169, 106052. https://doi.org/10.1016/j.apgeochem.2024.106052
- 46.
Yang, Z. Geochemical Characteristics of Geothermal Fluids in Chabu Geothermal Field, Xietongmen County, Tibet. Master Thesis, Tibet University, Lhasa, Tibet, China, 2022. https://doi.org/10.27735/d.cnki.gxzdx.2022.000039
- 47.
Rudnick, R.L.; Gao, S. 3.01 – Composition of the continental crust In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 1–64. https://doi.org/10.1016/B0-08-043751-6/03016-4
- 48.
London, D.; Morgan, G.B.; Icenhower, J. Stability and solubility of pollucite in the granite system at 200 MPa H2O. Can. Mineral. 1998, 36, 497–510. 10.3749/canmin.er00001
- 49.
Cerny, P.; London, D.; Novak, M. Granitic pegmatites as reflections of their sources. Elements 2012, 8, 289–294. https://doi.org/10.2113/gselements.8.4.289
- 50.
Saasen, A.; Jordal, O.H.; Burkhead, D.; et al. Drilling HT/HP wells using a cesium formate based drilling fluid. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, Dallas, TX, USA, 26–28 February 2002; Society of Petroleum Engineers (SPE): Amsterdam, The Netherlands, 2002; p SPE-74541-MS.
- 51.
Berg, P.C.; Pedersen, E.S.; Lauritsen, A˚ .; et al. Drilling and completing high-angle wells in high-density, cesium formate brine—The Kvitebjørn experience, 2004–2006. SPE Drill. Complet. 2009, 24, 15–24. https://doi.org/10.2118/112577-PA
- 52.
Jaduszliwer, B.; Camparo, J. Past, present and future of atomic clocks for GNSS. GPS Solut. 2021, 25, 27–39. https://doi.org/10.1007/s10291-020-01064-9
- 53.
Murakami, T.; Yamasaki, H. Plasma-fluid behavior of a less divergent disk magnetohydrodynamic generator using helium–cesium. IEEE Trans. Plasma Sci. 2004, 32, 1752–1759. https://doi.org/10.1109/TPS.2004.832551
- 54.
Wang, Z.; Wang, H.; Li, L.; et al. Combustion and plumeplasma characteristics of cesium-based solid propellant. Combust. Flame 2024, 263, 113419. https://doi.org/10.1016/j.combustflame.2024.113419
- 55.
Qiao, F.; Ma, X.; Qiu, X.; et al. Enhancing atomic layer deposition—microchannel plate gain with Cs activation in photoelectric devices. Opt. Eng. 2025, 64, 044102. https://doi.org/10.1117/1.OE.64.4.044102
- 56.
Sun, J.; Cai, Q.; Wan, Y.; et al. Promotional effects of cesium promoter on higher alcohol synthesis from syngas over Cspromoted Cu/ZnO/Al2O3 catalysts. ACS Catal. 2016, 6, 5771–5785. https://doi.org/10.1021/acscatal.6b01446
- 57.
Varala, R.; Achari, K.M.M.; Hussein, M.; et al. Cesium carbonate (Cs2CO3) in organic synthesis: A sexennial update (2018 to date). Curr. Org. Chem. 2025, 29, 2–18. https://doi.org/10.2174/1385272829666241009100505
- 58.
Melnikov, P.; Zanoni, L.Z. Clinical effects of cesium intake. Biol. Trace Elem. Res. 2010, 135, 355–363. https://doi.org/10.1007/s12011-009-8509-4
- 59.
Wang, R.C.; Hu, H.; Zhang, A.C.; et al. Pollucite and the cesium-dominant analogue of polylithionite as expressions of extreme Cs enrichment in the Yichun topaz–lepidolite granite, southern China. Can. Mineral. 2004, 42, 883–896. https://doi.org/10.2113/gscanmin.42.3.883
- 60.
Niu, H.; Yu, M.; Mubula, Y.; et al. Extraction of rubidium and cesium from a variety of resources: A review. Materials 2025, 18, 3378. https://doi.org/10.3390/ma18143378
- 61.
Yin, A. Cenozoic tectonic evolution of the Himalayan orogen as constrained by along-strike variation of structural geometry, exhumation history, and foreland sedimentation. Earth-Sci. Rev. 2006, 76, 1–131. https://doi.org/10.1016/j.earscirev.2005.05.004
- 62.
Yao, T.; Bolch, T.; Chen, D.; et al. The imbalance of the Asian water tower. Nat. Rev. Earth Environ. 2022, 3, 618–632. https://doi.org/10.1038/s43017-022-00299-4
- 63.
Li, S.; Zhao, S.; Liu, X.; et al. Closure of the Proto-Tethys Ocean and early Paleozoic amalgamation of microcontinental blocks in East Asia. Earth-Sci. Rev. 2018, 186, 37–75. https://doi.org/10.1016/j.earscirev.2017.01.011
- 64.
Ding, L.; Yang, D.; Cai, F.L.; et al. Provenance analysis of the Mesozoic Hoh-Xil-Songpan-Ganzi turbidites in northern Tibet: Implications for the tectonic evolution of the eastern Paleo-Tethys Ocean. Tectonics 2013, 32, 34–48. https://doi.org/10.1002/tect.20020
- 65.
Zhu, R.; Zhao, P.; Zhao, L. Tectonic evolution and geodynamics of the Neo-Tethys Ocean. Sci. China Earth Sci. 2022, 65, 1–24. https://doi.org/10.1007/s11430-021-9845-7
- 66.
Hou, Z.; Xu, B.; Zheng, Y.; et al. Mantle flow: The deep mechanism of large-scale growth in Tibetan Plateau. Chin. Sci. Bull. 2021, 66, 2671–2690. https://doi.org/10.1360/TB-2020-0817
- 67.
Nabelek, J.; Hetenyi, G.; Vergne, J.; et al. Underplating in the Himalaya–Tibet collision zone revealed by the Hi-CLIMB experiment. Science 2009, 325, 1371–1374. https://doi.org/10.1126/science.1167719
- 68.
Bian, S.; Gong, J.; Zuza, A.V.; et al. Along-strike variation in the initiation timing of the north-trending rifts in southern Tibet as revealed from the Yadong-Gulu rift. Tectonics 2022, 41, e2021TC007091. https://doi.org/10.1029/2021TC007091
- 69.
Tan, H.; Chen, X.; Shi, D.; et al. Base flow in the Yarlungzangbo River, Tibet, maintained by the isotopically-depleted precipitation and groundwater discharge. Sci. Total Environ. 2020, 743, 143510. https://doi.org/10.1016/j.scitotenv.2020.143510
- 70.
Liao, Z. Thermal Springs and Geothermal Energy in the Qinghai-Tibetan Plateau and the Surroundings; Springer: Cham, Switzerland, 2018.
- 71.
Tong, W.; Liao, Z.J.; Liu, S.B.; et al. Thermal Springs in Tibet; Beijing Science and Technology Press: Beijing, China, 2000.
- 72.
Chakrapani, G.J. Major and trace element geochemistry in Upper Ganga River in the Himalayas, India. Environ. Geol. 2005, 48, 189–201. https://doi.org/10.1007/s00254-005-1287-1
- 73.
Ollivier, P.; Radakovitch, O.; Hamelin, B. Major and trace element partition and fluxes in the Rhˆone River. Chem. Geol. 2011, 285, 15–31. https://doi.org/10.1016/j.chemgeo.2011.02.011
- 74.
DZ/T 0203–2002. People’s Republic of China Geological and Mineral Industry Standard: Specifications for Rare Metal Mineral Exploration; Standards Press of China: Beijing, China, 2002.
- 75.
Guo, Q.; Wang, Y. Geochemistry of hot springs in the Tengchong hydrothermal areas, southwestern China. J. Volcanol. Geotherm. Res. 2012, 215–216, 61–73. https://doi.org/10.1016/j.jvolgeores.2011.12.003
- 76.
Maity, J.P.; Chen, C.-Y.; Bundschuh, J.; et al. Hydrogeochemical reconnaissance of arsenic cycling and possible environmental risk in hydrothermal systems of Taiwan. Groundwater Sustain. Dev. 2017, 5, 1–13. https://doi.org/10.1016/j.gsd.2017.03.001
- 77.
Cullen, J.T.; Hurwitz, S.; Barnes, J.D.; et al. The systematics of chlorine, lihium, and boron and δ37Cl, δ7Li, and δ11B in the hydrothermal system of the yellowstone plateau volcanic field. Geochem. Geophys. Geosyst. 2021, 22, e2020GC009589. https://doi.org/10.1029/2020GC009589
- 78.
Bernard, R.; Taran, Y.; Pennisi, M.; et al. Chloride and boron behavior in fluids of Los Humeros geothermal field (Mexico): A model based on the existence of deep acid brine. Appl. Geochem. 2011, 26, 2064–2073. https://doi.org/10.1016/j.apgeochem.2011.07.004
- 79.
Cong, P.; Tan, H.; Shi, Z.; et al. Unusual boron isotopic value and hydrochemical characteristics of thermal springs indicating magmatic fluids upwelling along Cuona-Sangri Rift in the Tibet (China). Geothermics 2025, 127, 103222. https://doi.org/10.1016/j.geothermics.2024.103222
- 80.
Wei, S.; Zhang, W.; Fu, Y.; et al. Distribution characteristics and resource potential evaluation of lithium in geothermal water in China. Geol. China 2024, 51, 1527–1553. https://doi.org/10.12029/gc20230214001
- 81.
Zhu, H.; Tan, H.; Cong, P.; et al. Sources and enrichment mechanisms of Li-rich geothermal springs in the Mediterranean–Himalayan belt: Modelling from Li isotope and hydrochemistry. Geol. J. 2025, 60, 2019–2032. https://doi.org/10.1002/gj.5134
- 82.
Wang, C.; Zheng, M.; Zhang, X.; et al. O, H and Sr isotope evidence for origin and mixing processes of the Gudui geothermal system, Himalayas, China. Geosci. Front. 2020, 11, 1175–1187. https://doi.org/10.1016/j.gsf.2019.09.013
- 83.
Yuan, X.; Zhang, Y.; Huang, J.; et al. Hydrochemistry and multi-Isotopes for interpreting formation mechanisms of different-type geothermal waters in the Cuona-Woka Rift, Southern Tibetan plateau. Geosci. Front. 2025, 16, 102170. https://doi.org/10.1016/j.gsf.2025.102170
- 84.
Shi, Z.; Tan, H.; Cong, P.; et al. Speciation distribution and phase partition laws of rare alkali metals in geothermal Sinter deposits in Southern Tibet: Implications for the cesium mineralisation in the geothermal system. Chem. Geol. 2025, 692, 122975. https://doi.org/10.1016/j.chemgeo.2025.122975
- 85.
Qiang, K.; Tan, H.; Shi, Z.; et al. Study on the genetic relationship between siliceous rocks and surrounding cesium-rich siliceous Sinter in the Gudui Area of Xizang. Geol. Explor. 2025. https://doi.org/10.12134/j.dzykt.2025.06.023
- 86.
Zhou, Y.; Fu, W.; Yang, Z.; et al. Geochemical characteristics of Mesozoic chert from southern Tibet and its petrogenic implications. Acta Petrol. Sin. 2008, 24, 600–608. https://doi.org/10.18654/10000569/2008.03.600608.
- 87.
Tong, W.; Zhang, M.T.; Zhang, Z.F.; et al. Geothermals Beneath Xizang(Tibetan) Plateau; Beijing Science and Technology Press: Beijing, China, 1981.
- 88.
Pan, S.; Zhao, P.; Guan, H.; et al. Mechanisms of lithium and cesium enrichment in the semi-Dazi geothermal field, Qinghai-Xizang plateau: Insights from H–O–Li–Sr isotopes. Geotherm. Energy 2025, 13, 22. https://doi.org/10.1186/s40517-025-00348-3
- 89.
Millot, R.; Hegan, A.; N´egrel, P. Geothermal waters from the Taupo Volcanic zone, New Zealand: Li, B and Sr isotopes characterization. Appl. Geochem. 2012, 27, 677–688. https://doi.org/10.1016/j.apgeochem.2011.12.015
- 90.
Li, T.; Liu, J.-Q.; Wang, X.-H.; et al. Geochemical characteristics and genesis of gases from Tianchi Volcanic Springs. Changbai Mountains. Jilin, China. Bull. Mineral. Petrol. Geochem. 2015, 34, 1192–1202. https://doi.org/10.3969/j.issn.l007-2802.2015.06.011
- 91.
McDonough, W. F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. https://doi.org/10.1016/00092541(94)001404
- 92.
Hu, F.; Liu, X.; He, S.; et al. Cesium–rubidium mineralization in Himalayan leucogranites. Sci. China Earth Sci. 2023, 66, 2827–2852. https://doi.org/10.1007/s11430-022-1159-3
- 93.
Li, J.; Song, X. Tearing of Indian mantle lithosphere from highresolution seismic images and its implications for lithosphere coupling in Southern Tibet. Proc. Natl. Acad. Sci. USA 2018, 115, 8296–8300. https://doi.org/10.1073/pnas.1717258115
- 94.
Karakas, O.; Dufek, J.; Mangan, M.T.; et al. Thermal and petrologic constraints on lower crustal melt accumulation under the Salton sea geothermal field. Earth Planet. Sci. Lett. 2017, 467, 10–17. https://doi.org/10.1016/j.epsl.2017.02.027
- 95.
K¨uhn, C.; Brasse, H.; Schwarz, G. Three-dimensional electrical resistivity image of the volcanic arc in northern Chile—an appraisal of early magnetotelluric data. Pure Appl. Geophys. 2018, 175, 2153–2165.
- 96.
Wu, F.Y.; Liu, X.C.; Liu, Z.C.; et al. Highly fractionated Himalayan leucogranites and associated raremetal mineralization. Lithos 2020, 352–353, 105319. https://doi.org/10.1016/j.lithos.2019.105319
- 97.
Cao, H.-W.; Pei, Q.-M.; Santosh, M.; et al. Himalayan leucogranites: A review of geochemical and isotopic characteristics, timing of formation, genesis, and rare metal mineralization. Earth-Sci. Rev. 2022, 234, 104229. https://doi.org/10.1016/j.earscirev.2022.104229
- 98.
Tsumura, A.; Yamasaki, S. Background levels of trace elements in rain and river waters in Japan. Radioisotopes 1998, 47, 46–55. https://doi.org/10.3769/radioisotopes.47.46
- 99.
Klemm, L.M.; Pettke, T.; Heinrich, C.A. Fluid and source magma evolution of the Questa porphyry Mo deposit, New Mexico, USA. Miner. Depos. 2008, 43, 533–552. https://doi.org/10.1007/s00126-008-0181-7
- 100.
Yuan, Y.; Chen, B.; Shang, L.; et al. Lithium enrichment of magmatic–hydrothermal fluids in albite–spodumene pegmatite from Lijiagou, Eastern Tibetan Plateau: Evidence from fluid inclusions. Ore Geol. Rev. 2023, 162, 105685. https://doi.org/10.1016/j.oregeorev.2023.105685
- 101.
Tan, H.; Zhang, Y.; Zhang, W.; et al. Understanding the circulation of geothermal waters in the Tibetan Plateau using oxygen and hydrogen stable isotopes Appl. Geochem. 2014, 51, 23–32. https://doi.org/10.1016/j.apgeochem.2014.09.006
- 102.
Yang, L.; Wang, J.-M.; Liu, X.-C.; et al. Petrogenetic link between leucogranite and spodumene pegmatite in Lhozhag, eastern Himalaya: Constraints from U–(Th)–Pb geochronology and Li–Nd–Hf isotopes. Lithos 2024, 470–471, 107530. https://doi.org/10.1016/j.lithos.2024.107530
- 103.
Zhao, H.; Chen, B.; Huang, C.; et al. Geochemical and Sr–Nd–Li isotopic constraints on the genesis of the Jiajika Li-rich pegmatites, eastern Tibetan Plateau: Implications for Li mineralization. Contrib. Mineral. Petrol. 2021, 177, 4. https://doi.org/10.1007/S00410-021-01869-3
- 104.
Gao, P.; Zheng, Y.-F.; Mayne, M.J.; et al. Miocene high-temperature leucogranite magmatism in the Himalayan orogen. GSA Bull. 2020, 133, 679–690. https://doi.org/10.1130/B35691.1
- 105.
Xie, L.; Tao, X.; Wang, R.; et al. Highly fractionated leucogranites in the eastern Himalayan Cuonadong dome and related magmatic Be–Nb–Ta and hydrothermal Be–W–Sn mineralization. Lithos 2020, 354–355, 105286. https://doi.org/10.1016/j.lithos.2019.105286
- 106.
Fan, J.-J.; Tang, G.-J.;Wei, G.-J.; et al. Lithium isotope fractionation during fluid exsolution: Implications for Li mineralization of the Bailongshan pegmatites in the West Kunlun, NW Tibet. Lithos 2020, 352, 105236. https://doi.org/10.1016/j.lithos.2019.105236
- 107.
Troch, J.; Huber, C.; Bachmann, O. The physical and chemical evolution of magmatic fluids in near-solidus silicic magma reservoirs: Implications for the formation of pegmatites. Am. Mineral. 2022, 107, 190–205. https://doi.org/10.2138/am-2022-7997
- 108.
Thomas, R.; Davidson, P.; Badanina, E. A melt and fluid inclusion assemblages in beryl from pegmatite in the Orlovka amazonite granite, East Transbaikalia, Russia: Implications for pegmatite-forming melt systems. Miner. Petrol. 2009, 96, 129–140. https://doi.org/10.1007/s00710-009-0053-6
- 109.
Wang, G.; Liu, Y.; Zhu, X.; et al. The status and development trend of geothermal resources in China. Earth Sci. Front. 2020, 27, 191–201. https://doi.org/10.13745/j.esf.sf.2020.1.1
- 110.
Casey, W.H.; Sposito, G. On the temperature dependence of mineral dissolution rates. Geochim. Cosmochim. Acta 1992, 56, 3825–3830. https://doi.org/10.1016/0016-7037(92)90173-G
- 111.
Walter, M.R.; Desmarais, D.; Farmer, J.D.; et al. Lithofacies and biofacies of mid-Paleozoic thermal spring deposits in the Drummond Basin, Queensland, Australia. PALAIOS 1996, 11, 497–518. https://doi.org/10.2307/3515199
- 112.
De Filippis, L.; Faccenna, C.; Billi, A.; et al. Plateau versus Fissure ridge travertines from Quaternary geothermal springs of Italy and Turkey: Interactions and feedbacks between fluid discharge, paleoclimate, and tectonics. Earth-Sci. Rev. 2013, 123, 35–52. https://doi.org/10.1016/j.earscirev.2013.04.001
- 113.
Djokic, T.; Bolhar, R.; Brengman, L. A.; et al. Trace elements (REE + Y) reveal marine, subaerial, and hydrothermal controls on an early Archean habitat for life: The 3.48 Ga volcanic-caldera system of the dresser formation, Pilbara Craton. Chem. Geol. 2024, 644, 121865. https://doi.org/10.1016/j.chemgeo.2023.121865
- 114.
McKenzie, E.J.; Brown, K.L.; Cady, S.L.; et al. Trace metal chemistry and silicification of microorganisms in geothermal Sinter, Taupo Volcanic Zone, New Zealand. Geothermics 2001, 30, 483–502. https://doi.org/10.1016/S0375-6505(01)00006-9
- 115.
Hartman, I.E.; Tan, H.; Shi, D.; et al. Elemental distribution and partitioning law between the geothermal water and associated deposits for a typical geothermal system with large-scale siliceous sinter deposits in the Tibet. Geochem. Int. 2021, 59, 1258–1273. https://doi.org/10.1134/S0016702921130032
- 116.
Baek, W.; Avramov, P.V.; Kim, Y. Nuclear magnetic resonance and theoretical simulation study on Cs ion Co-adsorbed with other alkali cations on illite. Appl. Surf. Sci. 2019, 489, 766–775. https://doi.org/10.1016/j.apsusc.2019.06.039
- 117.
Boudreau, A.E.; Lynne, B.Y. The growth of siliceous sinter deposits around high-temperature eruptive hot springs. J. Volcanol. Geotherm. Res. 2012, 247–248, 1–8. https://doi.org/10.1016/j.jvolgeores.2012.07.008
- 118.
Zhou, B.; Ren, E.; Sherriff, B.L.; et al. Multinuclear NMR study of Cs-bearing geyserites of the Targejia hot spring cesium deposit in Tibet. Am. Mineral. 2013, 98, 907–913. https://doi.org/10.2138/am.2013.4321
- 119.
Liu, R.; Liu, F.; Hu, C.; et al. Simultaneous removal of Cd(II) and Sb(V) by Fe–Mn binary oxide: Positive effects of Cd(II) on Sb(V) adsorption. J. Hazard. Mater. 2015, 300, 847–854. https://doi.org/10.1016/j.jhazmat.2015.08.010
- 120.
Yang, K.; Liu, Y.; Li, Y.; et al. Applications and characteristics of Fe–Mn binary oxides for Sb(V) removal in textile wastewater: Selective adsorption and the fixed-bed column study. Chemosphere 2019, 232, 254–263. https://doi.org/10.1016/j.chemosphere.2019.05.174
- 121.
Campbell, K.A.; Guido, D.M.; Gautret, P.; et al. Geyserite in hotspring siliceous sinter: Window on Earth’s hottest terrestrial (Paleo) Environment and its extreme life. Earth-Sci. Rev. 2015, 148, 44–64. https://doi.org/10.1016/j.earscirev.2015.05.003
- 122.
Dobson, P.; Araya, N.; Brounce, M.; et al. Characterizing the Geothermal Lithium Resource at the Salton Sea; Lawrence Berkeley National Laboratory (LBNL): Berkeley, CA, USA, 2023. https://www.osti.gov/biblio/2222403 (accessed on 3 November 2024)
- 123.
Gao, L.; Ma, G.; Zheng, Y.; et al. Research trends on separation and extraction of rare alkali metal from salt lake brine: Rubidium and cesium. Solvent Extr. Ion Exch. 2020, 38, 753–776. https://doi.org/10.1080/07366299.2020.1802820
- 124.
Bolinger, M.; Millstein, D.; Gorman, W.; et al. Mind the gap: Comparing the net value of geothermal, wind, solar, and solar+ storage in the western United States. Renew. Energy 2023, 205, 999–1009. https://doi.org/10.1016/j.renene.2023.01.087
- 125.
Ricks, W.; Voller, K.; Galban, G.; et al. The role of flexible geothermal power in decarbonized electricity systems. Nat. Energy 2025, 10, 28–40. https://doi.org/10.1038/s41560-024-01635-5
- 126.
Pan, S.-Y.; Gao, M.; Shah, K.J.; et al. Establishment of enhanced geothermal energy utilization plans: Barriers and strategies. Renew. Energy 2019, 132, 19–32. https://doi.org/10.1016/j.renene.2018.07.036
- 127.
Tester, J.W.; Beckers, K.F.; Hawkins, A.J.; et al. The evolving role of geothermal energy for decarbonizing the United States Energy Environ. Sci. 2021, 14, 6211–6241. https://doi.org/10.1039/D1EE02043F
- 128.
Vargas, C.A.; Caracciolo, L.; Ball, P.J. Geothermal energy as a means to decarbonize the energy mix of megacities Commun. Earth Environ. 2022, 3, 66. https://doi.org/10.1038/s43247-022-00385-8
- 129.
Wei, W.; Ge, Z.; Geng, Y.; et al. Toward carbon neutrality: Uncovering constraints on critical minerals in the Chinese power system. Fundam. Res. 2022, 2, 367–377. https://doi.org/10.1016/j.fmre.2022.01.005
- 130.
Shi, H.; Heng, J.; Duan, H.; et al. Critical mineral constraints pressure energy transition and trade toward the Paris Agreement climate goals. Nat. Commun. 2025, 16, 4496. https://doi.org/10.1038/s41467-025-4496-4
- 131.
Mousavinezhad, S.; Nili, S.; Fahimi, A.; et al. Environmental impact assessment of direct lithium extraction from brine resources: Global warming potential, land use, water consumption, and charting sustainable scenarios. Resour. Conserv. Recycl. 2024, 205, 107583. https://doi.org/10.1016/j.resconrec.2024.107583
- 132.
Zhu, Y.; Hu, Y.; Zhu, Y. Can China’s energy policies achieve the “dual carbon” goal? A multi-dimensional analysis based on policy text tools. Environ. Dev. Sustain. 2024, 26, 1–40. https://doi.org/10.1007/s10668-024-04637-3

This work is licensed under a Creative Commons Attribution 4.0 International License.


