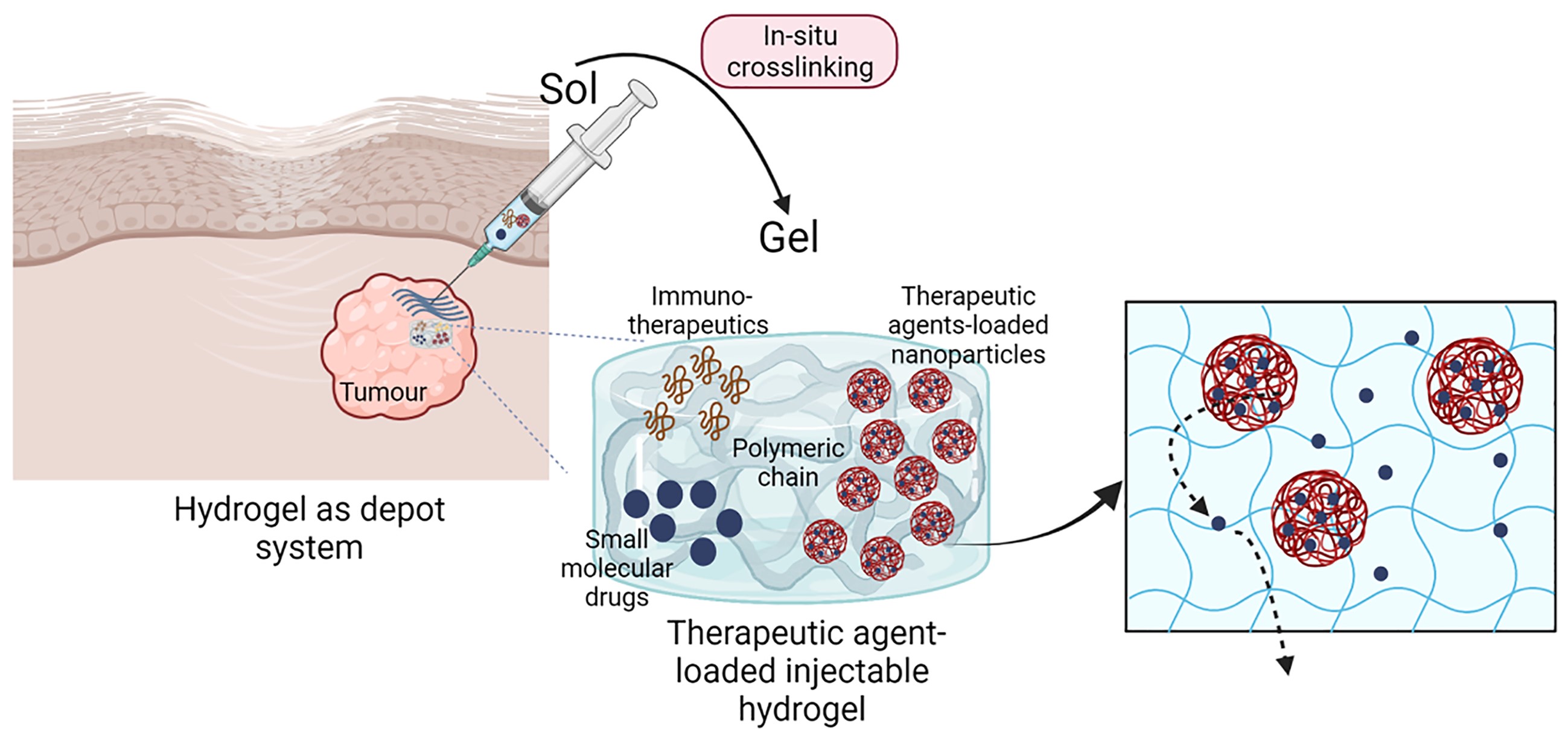
Hydrogels are water-based polymeric three-dimensional network with advantageous properties for the delivery of bioactive components, ranging from small therapeutic agents to therapeutic cells. Natural-based hydrogels have great potential as delivery vehicles for the local controlled release of therapeutic agents at the target site. Injectable hydrogels are designed to load therapeutic agents by simple mixing within the polymer solutions, as well as use nanoparticles able to respond to specific external conditions, such as temperature and pH. Herein, we present an overview of the properties of natural injectable hydrogels and recent developments for their use to control the local release of therapeutic agents; as well as strategies to crosslink in-situ multifunctional injectable hydrogels that act as therapeutical depot system. The mini review focuses on alginate-based injectable hydrogels as controlled drug delivery systems, presenting advantages and challenges of their application in cancer therapy
- Open Access
- Mini Review
Injectable Multifunctional Natural Polymer-Based Hydrogels for the Local Delivery of Therapeutic Agents
Author Information
Received: 13 Nov 2022 | Accepted: 15 Dec 2022 | Published: 21 Dec 2022
Abstract
Graphical Abstract
Keywords
alginate | injectable hydrogels | local drug delivery | natural polymers
References
- 1.Bhattarai N.; Gunn J.; Zhang M.Q. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Delivery Rev., 2010, 62(1): 83-99.
- 2.Bai X.; Smith Z.L.; Wang Y.H.; et al. Sustained drug release from smart nanoparticles in cancer therapy: a comprehensive review. Micromachines, 2022, 13(10): 1623.
- 3.Anselmo A.C.; Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J. Controlled Release, 2014, 190: 15-28.
- 4.Ahmed E.M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res., 2015, 6(2): 105-121.
- 5.Dimatteo R.; Darling N.J.; Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Delivery Rev., 2018, 127: 167-184.
- 6.Obara K.; Ishihara M.; Ozeki Y.; et al. Controlled release of paclitaxel from photocrosslinked chitosan hydrogels and its subsequent effect on subcutaneous tumor growth in mice. J. Controlled Release, 2005, 110(1): 79-89.
- 7.Bos G.W.; Jacobs J.J.L.; Koten J.W.; et al. In situ crosslinked biodegradable hydrogels loaded with IL-2 are effective tools for local IL-2 therapy. Eur. J. Pharm. Sci., 2004, 21(4): 561-567.
- 8.Thambi T.; Li Y.; Lee D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Controlled Release, 2017, 267: 57-66.
- 9.Clarivate. Web of science core collection. (accessed on 4 November 4 2022). https://clarivate.com/products/scientific-and-academic-research/research-discovery-and-workflow-solutions/web-of-science/web-of-science-core-collection/.
- 10.Tanan W.; Panichpakdee J.; Saengsuwan S. Novel biodegradable hydrogel based on natural polymers: synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J., 2019, 112: 678-687.
- 11.Zhao W.; Jin X.; Cong Y.; et al. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol., 2013, 88(3): 327-339.
- 12.Akindoyo J.O.; Mariatti M.; Hamid Z.A.A.; et al. Injectable hydrogel scaffold from natural biomaterials - An overview of recent studies. AIP Conf. Proc., 2020, 2267: 020068.
- 13.Lee K.Y.; Mooney D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci., 2012, 37(1): 106-126.
- 14.Bello A.B.; Kim D.; Kim D.; et al. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng., Part B, 2020, 26(2): 164-180.
- 15.Tang S.S.; Mohad V.; Gowda M.; et al. Insights into the role of collagen in vocal fold health and disease. Journal of Voice, 2017, 31(5): 520-527.
- 16.Bakshi P.S.; Selvakumar D.; Kadirvelu K.; et al. Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. Int. J. Biol. Macromol., 2020, 150: 1072-1083.
- 17.Abatangelo G.; Vindigni V.; Avruscio G.; et al. Hyaluronic acid: redefining its role. Cells, 2020, 9(7): 1743.
- 18.Aravamudhan A.; Ramos D.M.; Nada A.A.; et al. Chapter 4 — Natural polymers: polysaccharides and their derivatives for biomedical applications. Kumbar S.G.; Laurencin C.T.; Deng M. Natural and synthetic biomedical polymers. Oxfor: Elsevier, 2014: 67-89.
- 19.Varghese S.A.; Rangappa S.M.; Siengchin S.; et al. Chapter 2 — Natural polymers and the hydrogels prepared from them. Chen Y. Hydrogels Based on Natural Polymers. Amsterdam: Elsevier, 2020: 17-47.
- 20.Sun W.Z.; Gregory D.A.; Tomeh M.A.; et al. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci., 2021, 22(3): 1499.
- 21.Tong Z.R.; Jin L.L.; Oliveira J.M.; et al. Adaptable hydrogel with reversible linkages for regenerative medicine: dynamic mechanical microenvironment for cells. Bioact. Mater., 2021, 6(5): 1375-1387.
- 22.Fakhari A.; Subramony J.A. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J. Controlled Release, 2015, 220, Part A: 465-475.
- 23.Marques A.C.; Costa P.J.; Velho S.; et al. Stimuli-responsive hydrogels for intratumoral drug delivery. Drug Discovery Today, 2021, 26(10): 2397-2405.
- 24.Ma Q.; Li Q.; Cai X.; et al. Injectable hydrogels as drug delivery platform for in-situ treatment of malignant tumor. J. Drug Delivery Sci. Technol., 2022, 76: 103817.
- 25.Zhao J.; Zhao X.; Guo B.L.; et al. Multifunctional interpenetrating polymer network hydrogels based on methacrylated alginate for the delivery of small molecule drugs and sustained release of protein. Biomacromolecules, 2014, 15(9): 3246-3252.
- 26.Ono K.; Saito Y.; Yura H.; et al. Photocrosslinkable chitosan as a biological adhesive. J. Biomed. Mater. Res., 2000, 49(2): 289-295.
- 27.Emoto S.; Yamaguchi H.; Kamei T.; et al. Intraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg. Today, 2014, 44(5): 919-926.
- 28.Trombino S.; Servidio C.; Curcio F.; et al. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics, 2019, 11(8): 407.
- 29.Andrade F.; Roca-Melendres M.M.; Durán-Lara E.F.; et al. Stimuli-responsive hydrogels for cancer treatment: the role of pH, light, ionic strength and magnetic field. Cancers, 2021, 13(5): 1164.
- 30.Ruel-Gariépy E.; Shive M.; Bichara A.; et al. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm., 2004, 57(1): 53-63.
- 31.Qu J.; Zhao X.; Ma P.X.; et al. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater., 2017, 58: 168-180.
- 32.Zhao L.L.; Zhu L.J.; Liu F.Y.; et al. pH triggered injectable amphiphilic hydrogel containing doxorubicin and paclitaxel. Int. J. Pharm., 2011, 410(1/2): 83-91.
- 33.Iravani S.; Varma R.S. Alginate-based micro- and nanosystems for targeted cancer therapy. Mar. Drugs, 2022, 20(10): 598.
- 34.Chao Y.; Liang C.; Tao H.Q.; et al. Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses. Sci. Adv., 2020, 6(10): eaaz4204.
- 35.Ferreira N.N.; Ferreira L.M.B.; Miranda-Gonçalves V.; et al. Alginate hydrogel improves anti-angiogenic bevacizumab activity in cancer therapy. Eur. J. Pharm. Biopharm., 2017, 119: 271-282.
- 36.Alamzadeh Z.; Beik J.; Mirrahimi M.; et al. Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur. J. Pharm. Sci., 2020, 145: 105235.
- 37.Xiao T.T.; Zhu J.Z.; Jia L.; et al. Injectable alginate hydrogels for synergistic tumor combination therapy through repolarization of tumor-associated macrophages. J. Controlled Release, 2022, 348: 239-249.
- 38.Mandal A.; Clegg J.R.; Anselmo A.C.; et al. Hydrogels in the clinic. Bioeng. Transl. Med., 2020, 5(2): e10158.
How to Cite
Bai, X.; Tirella, A. Injectable Multifunctional Natural Polymer-Based Hydrogels for the Local Delivery of Therapeutic Agents. International Journal of Drug Discovery and Pharmacology 2022, 1 (1), 10. https://doi.org/10.53941/ijddp.v1i1.203.
RIS
BibTex
Copyright & License

Annalisa Tirella, Xue Bai
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


