We focus here on the Hippo pathway in the hierarchical sensing and modulation of the mechanical state of the adult heart in health and disease. The Hippo pathway interrogates the micro-environment of cardiac myocytes providing surveillance of the mechanical state with engagement of signaling pathways critical to homeostasis of cardiac development, remodeling, and function and vulnerable to pathologies. Our discussion centers on Hippo signaling in the altered mechanical state instigated by variants of genes expressing mutant sarcomere proteins that trigger a progression to dilated cardiomyopathy (familial DCM). There is an unmet need for therapies in DCM. Recent progress in the discovery of small molecules that target Hippo signaling and are intended for use in cardiac disorders provides leads for modifying Hippo in DCM. As we emphasize, identifying useful targets in DCM requires in depth understanding of cell specific Hippo signaling in the cardiac micro-environment.
- Open Access
- Review
The Hippo Signaling Pathway as a Drug Target in Familial Dilated Cardiomyopathy
- Paulina Langa 1,
- Beata M. Wolska 1, 2,
- R. John Solaro 1, *
Author Information
Received: 01 Nov 2022 | Accepted: 24 Nov 2022 | Published: 21 Dec 2022
Abstract
Graphical Abstract
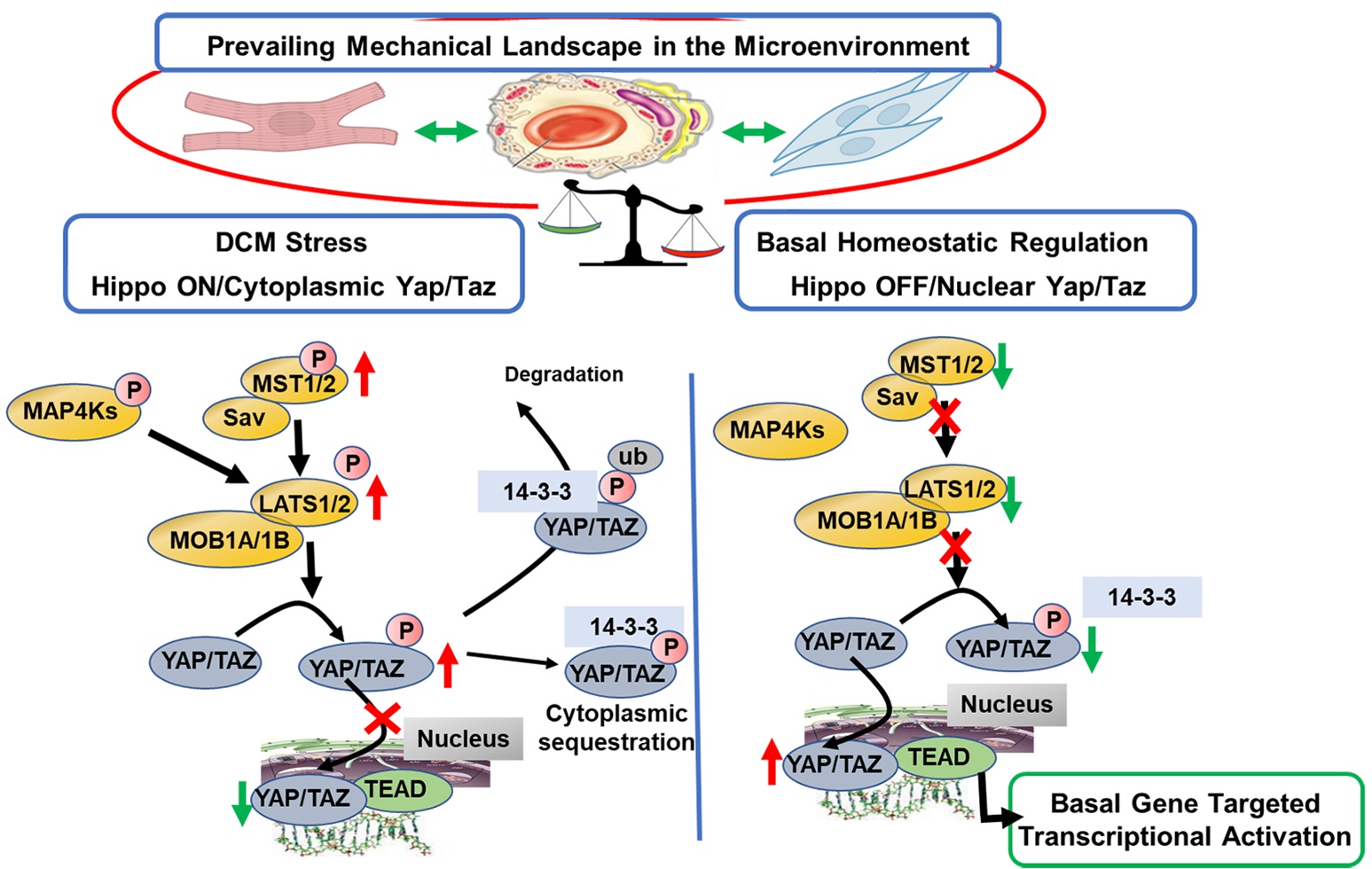
Keywords
mutant sarcomere proteins | coronary vasculature | endothelium | fibroblasts | extra-cellular matrix
References
- 1.Tucker N.R.; Chaffin M.; Fleming S.J.; et al. Transcriptional and cellular diversity of the human heart. Circulation, 2020, 142(5): 466-482.
- 2.Alves M.L.; Dias F.A.L.; Gaffin R.D.; et al. Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ.: Cardiovasc. Genet., 2014, 7(2): 132-143.
- 3.Keam S.J. Mavacamten: first approval. Drugs, 2022, 82(10): 1127-1135.
- 4.Weintraub R.G.; Semsarian C.; Macdonald P. Dilated cardiomyopathy. Lancet, 2017, 390(10092): 400-414.
- 5.Clippinger S.R.; Cloonan P.E.; Greenberg L.; et al. Disrupted mechanobiology links the molecular and cellular phenotypes in familial dilated cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A., 2019, 116(36): 17831-17840.
- 6.Alves M.L.; Warren C.M.; Simon J.N.; et al. Early sensitization of myofilaments to Ca2+ prevents genetically linked dilated cardiomyopathy in mice. Cardiovasc. Res., 2017, 113(8): 915-925.
- 7.Puglisi J.L.; Goldspink P.H.; Gomes A.V.; et al. Influence of a constitutive increase in myofilament Ca2+-sensitivity on Ca2+-fluxes and contraction of mouse heart ventricular myocytes. Arch. Biochem. Biophys., 2014, 552/553: 50-59.
- 8.Carley A.N.; Taglieri D.M.; Bi J.; et al. Metabolic efficiency promotes protection from pressure overload in hearts expressing slow skeletal troponin I. Circ.: Heart Failure, 2015, 8(1): 119-127.
- 9.Utter M.S.; Ryba D.M.; Li B.H.; et al. Omecamtiv mecarbil, a cardiac myosin activator, increases Ca2+ sensitivity in myofilaments with a dilated cardiomyopathy mutant tropomyosin E54K. J. Cardiovasc. Pharmacol., 2015, 66(4): 347-353.
- 10.Morgan B.P.; Muci A.; Lu P.P.; et al. Discovery of omecamtiv mecarbil the first, selective, small molecule activator of cardiac myosin. ACS Med. Chem. Lett., 2010, 1(9): 472-477.
- 11.Del Re D.P. Hippo signaling in the heart - non-canonical pathways impact growth, survival and function. Circ. J., 2016, 80(7): 1504-1510.
- 12.Cho Y.S.; Jiang J. Hippo-independent regulation of Yki/Yap/Taz: a non-canonical view. Frontiers in Cell and Developmental Biology, 2021, 9: 658481.
- 13.Ikeda S.; Sadoshima J. Regulation of myocardial cell growth and death by the Hippo pathway. Circ. J., 2016, 80(7): 1511-1519.
- 14.Xie J.H.; Wang Y.X.; Ai D.; et al. The role of the Hippo pathway in heart disease. FEBS J., 2022, 289(19): 5819-5833.
- 15.Wang J.; Liu S.J.; Heallen T.; et al. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol., 2018, 15(11): 672-684.
- 16.Zheng A.C.; Chen Q.S.; Zhang L. The Hippo-YAP pathway in various cardiovascular diseases: focusing on the inflammatory response. Front. Immunol., 2022, 13: 971416.
- 17.Adler J.J.; Johnson D.E.; Heller B.L.; et al. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of angiomotin by the LATS1/2 protein kinases. Proc. Natl. Acad. Sci. U. S. A., 2013, 110(43): 17368-17373.
- 18.You B.; Yan G.J.; Zhang Z.L.; et al. Phosphorylation of cardiac troponin I by mammalian sterile 20-like kinase 1. Biochem. J., 2009, 418(1): 93-101.
- 19.Odashima M.; Usui S.; Takagi H.; et al. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ. Res., 2007, 100(9): 1344-1352.
- 20.Yamamoto S.; Yang G.P.; Zablocki D.; et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J. Clin. Invest., 2003, 111(10): 1463-1474.
- 21.Chan S.W.; Lim C.J.; Chong Y.F.; et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J. Biol. Chem., 2011, 286(9): 7018-7026.
- 22.Liu R.Y.; Lee J.; Kim B.S.; et al. Tead1 is required for maintaining adult cardiomyocyte function, and its loss results in lethal dilated cardiomyopathy. JCI Insight., 2017, 2(17): e93343.
- 23.Hou N.; Wen Y.; Yuan X.; et al. Activation of Yap1/Taz signaling in ischemic heart disease and dilated cardiomyopathy. Exp. Mol. Pathol., 2017, 103(3): 267-275.
- 24.Wu W.; Ziemann M.; Huynh K.; et al. Activation of Hippo signaling pathway mediates mitochondria dysfunction and dilated cardiomyopathy in mice. Theranostics, 2021, 11(18): 8993-9008.
- 25.Nguyen M.N.; Ziemann M.; Kiriazis H.; et al. Galectin-3 deficiency ameliorates fibrosis and remodeling in dilated cardiomyopathy mice with enhanced Mst1 signaling. Am. J. Physiol.: Heart Circ. Physiol., 2019, 316(1): H45-H60.
- 26.Xiao Y.; Hill M.C.; Li L.L.; et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes Dev., 2019, 33(21/22): 1491-1505.
- 27.Weisleder N.; Soumaka E.; Abbasi S.; et al. Cardiomyocyte-specific desmin rescue of desmin null cardiomyopathy excludes vascular involvement. J. Mol. Cell. Cardiol., 2004, 36(1): 121-128.
- 28.Heusch G. Coronary blood flow in heart failure: cause, consequence and bystander. Basic Res. Cardiol., 2022, 117(1): 1.
- 29.Park J.A.; Kwon Y.G. Hippo-YAP/TAZ signaling in angiogenesis. BMB Rep., 2018, 51(3): 157-162.
- 30.Ragni C.V.; Diguet N.; Le Garrec J.F.; et al. Amotl1 mediates sequestration of the Hippo effector Yap1 downstream of Fat4 to restrict heart growth. Nat. Commun., 2017, 8: 14582.
- 31.Kastan N.; Gnedeva K.; Alisch T.; et al. Small-molecule inhibition of Lats kinases may promote Yap-dependent proliferation in postmitotic mammalian tissues. Nat. Commun., 2021, 12(1): 3100.
- 32.Fan F.Q.; He Z.X.; Kong L.L.; et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci. Transl. Med., 2016, 8(352): 352ra108.
- 33.Ikeda S.; Mizushima W.; Sciarretta S.; et al. Hippo deficiency leads to cardiac dysfunction accompanied by cardiomyocyte dedifferentiation during pressure overload. Circ. Res., 2019, 124(2): 292-305.
- 34.Kashihara T.; Sadoshima J. Role of YAP/TAZ in energy metabolism in the heart. J. Cardiovasc. Pharmacol., 2019, 74(6): 483-490.
- 35.Del Re D.P. Beyond the cardiomyocyte: consideration of HIPPO pathway cell-type specificity. Circ. Res., 2018, 123(1): 30-32.
- 36.Heiman M.; Kulicke R.; Fenster R.J.; et al. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat. Protoc., 2014, 9(6): 1282-1291.
- 37.Lou J.F.; Lu Y.H.; Cheng J.; et al. A chemical perspective on the modulation of TEAD transcriptional activities: recent progress, challenges, and opportunities. Eur. J. Med. Chem., 2022, 243: 114684.
- 38.Cunningham R.; Hansen C.G. The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clin. Sci., 2022, 136(3): 197-222.
- 39.Ky B.; Vejpongsa P.; Yeh E.T.H.; et al. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ. Res., 2013, 113(6): 754-764.
- 40.Dobbin S.J.H.; Cameron A.C.; Petrie M.C.; et al. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. Heart, 2018, 104(24): 1995-2002.
How to Cite
Langa, P.; Wolska, B. M.; Solaro, R. J. The Hippo Signaling Pathway as a Drug Target in Familial Dilated Cardiomyopathy. International Journal of Drug Discovery and Pharmacology 2022, 1 (1), 4. https://doi.org/10.53941/ijddp.v1i1.189.
RIS
BibTex
Copyright & License

R. John Solaro, Beata M. Wolska, Paulina Langa
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


