Currently, with an increased requirement for new therapeutic strategies, preclinical drug testing or screening platforms have rapidly evolved in recent years. In comparison to traditional 2D cell cultures, 3D organoids or spheroids with or without scaffolds improve the microenvironment of in vitro cultures, advancing the in vitro biological observation and enabling mechanistic studies of drug reactions in the human tissue-like environment. 3D organoids and spheroids are straightforward to produce, and relatively uniform in size and shape. This helps to facilitate high throughput screening requirements. Spheroids and organoids have been applied in anti-cancer drug testing, toxicity evaluations, as well as mechanism studies for variable organ systems, including the intestine, liver, pancreas, brain, and heart. Among 3D cultures of spheroids and organoids, ‘tumour spheroids’ formed by dissociated tumour tissues or cancer cell lines are relatively simple in composition and commonly applied to anticancer drug screening. The ‘healthy organoids’ differentiated from hiPSCs/hESCs are more complex in cell composition, distribution, structure and function with higher similarity to in vivo organs, and have found applications in toxicity tests, personalised medicine, and therapeutic and mechanistic studies. In most cases, the multicellular 3D organoids are more resistant and stable in reaction to stimulations or chemicals in vitro , suggesting more accurate modelling of in vivo responses. Here, we review recent progress in human-origin organoid/spheroid systems and their applications in preclinical studies.
- Open Access
- Review
Progress of 3D Organoid Technology for Preclinical Investigations: Towards Human In Vitro Models
- Yingjuan Liu *,
- Honglin Xu,
- Sabu Abraham,
- Xin Wang,
- Bernard D. Keavney *
Author Information
Received: 01 Nov 2022 | Accepted: 30 Nov 2022 | Published: 21 Dec 2022
Abstract
Graphical Abstract
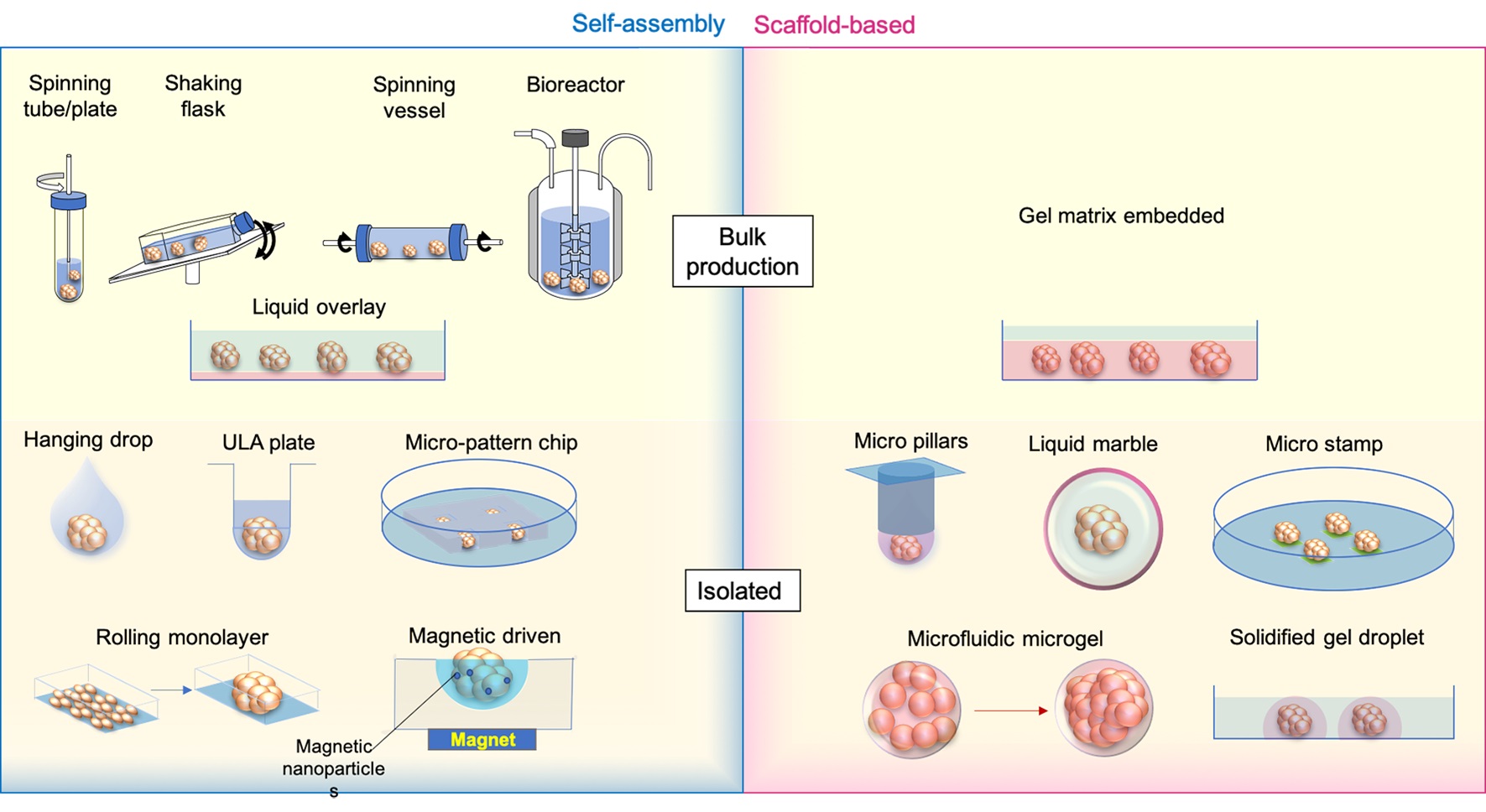
Keywords
drug screening | disease modelling | preclinical application | self-assembly | self-organising | 3D culture
References
- 1.Jaroch K.; Jaroch A.; Bojko B. Cell cultures in drug discovery and development: The need of reliable in vitro-in vivo extrapolation for pharmacodynamics and pharmacokinetics assessment. J. Pharm. Biomed. Anal., 2018, 147:297-312, doi: 10.1016/j.jpba.2017.07.023.
- 2.Imamura Y.; Mukohara T.; Shimono Y.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep., 2015, 33(4):1837-43, doi: 10.3892/or.2015.3767.
- 3.Nowacka M.; Sterzynska K.; Andrzejewska M.; et al. Drug resistance evaluation in novel 3D in vitro model. Biomed. Pharmacother., 2021, 138:111536, doi: 10.1016/j.biopha.2021.111536.
- 4.Roper S.J.; Linke F.; Scotting PJ.; et al. 3D spheroid models of paediatric SHH medulloblastoma mimic tumour biology, drug response and metastatic dissemination. Sci. Rep., 2021, 11(1):4259, doi: 10.1038/s41598-021-83809-6.
- 5.Li S.; Yang K.; Chen X.; et al. Simultaneous 2D and 3D cell culture array for multicellular geometry, drug discovery and tumor microenvironment reconstruction. Biofabrication, 2021, 13(4), doi: 10.1088/1758-5090/ac1ea8.
- 6.Fontoura J.C.; Viezzer C.; dos Santos F.G.; et al. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C. Mater. Biol. Appl., 2020,107:110264, doi:10.1016/j.msec.2019.110264.
- 7.Edmondson R.; Broglie J.J.; Adcock A.F.; et al. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol., 2014, 12(4):207-18, doi:10.1089/adt.2014.573.
- 8.Kapałczyńska M.; Kolenda T.; Przybyła W.; et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch. Med. Sci., 2018,14(4):910-9, doi: 10.3390/ijms222212200.
- 9.Wilson H. A new method by which sponges may be artificially reared. Science, 1907, 25(649):912-5, doi: 10.1126/science.25.649.912, doi: 10.1126/science.25.649.912.
- 10.Steinberg M.S. The problem of adhesive selectivity in cellular interactions. Cellular membranes in development. 22: Academic Press New York; 1964. p. 321-66.
- 11.James A.W.; Jennifer J.; Swiergiel V.S.M.; et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science, 1998, 282(5391):1145, doi: 10.1126/science.282.5391.1145.
- 12.Shannon J.M.; Mason R.J.; Jennings S.D. Functional differentiation of alveolar type II epithelial cells in vitro: effects of cell shape, cell-matrix interactions and cell-cell interactions. Biochim. Biophys. Acta., 1987, 931(2):143-156, doi:10.1016/0167-4889(87)90200-x.
- 13.Pollard R.T.; Salter I.; Sanders R.J.; et al. Southern Ocean deep-water carbon export enhanced by natural iron fertilization. Nature, 2009, 457(7229):577-80, doi:10.1038/nature07716 .
- 14.Corrò C.; Novellasdemunt L.; Li V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol., 2020, 319(1):C151-C65, doi:10.1152/ajpcell.00120.2020.
- 15.Huch M.; Knoblich J.A.; Lutolf M.P.; et al. The hope and the hype of organoid research. Development. 2017,144(6):938-41, doi: 10.1242/dev.150201.
- 16.Xu H.; Jiao Y.; Qin S.; et al. Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp. Hematol. Oncol., 2018,7:30, doi: 10.1186/s40164-018-0122-9.
- 17.Karolak A.; Poonja S.; Rejniak K.A. Morphophenotypic classification of tumor organoids as an indicator of drug exposure and penetration potential. PLOS Computational Biology. 2019,15(7):e1007214, doi: 10.1186/s40164-018-0122-9.
- 18.Gunti S.; Hoke A.T.K.; Vu K.P.; et al. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers (Basel). 2021,13(4), doi: 10.3390/cancers13040874.
- 19.Sutherland R.M.; McCredie J.A.; Inch W.R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst., 1971,46(1):113-20.
- 20.Sutherland R.M.; Inch W.R.; McCredie J.A.; et al. A multi-component radiation survival curve using an in vitro tumour model. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 1970,18(5):491-5.
- 21.Sakalem M.E.; De Sibio M.T.; da Costa F.A.d.S.; et al. Historical evolution of spheroids and organoids, and possibilities of use in life sciences and medicine. J. Biotechnology. 2021,16(5):2000463, https://doi.org/10.1002/biot.202000463.
- 22.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J. Biosci. Bioeng., 2007;103(5):389-398. doi:10.1263/jbb.103.389.
- 23.Liyang G.; Abdullah S.; Rosli R; et al. Neural Commitment of Embryonic Stem Cells through the Formation of Embryoid Bodies (EBs). Malays. J. Med. Sci., 2014,21(5):8-16.
- 24.Imamura T.; Cui L.; Teng R.; et al. Embryonic stem cell-derived embryoid bodies in three-dimensional culture system form hepatocyte-like cells in vitro and in vivo. Tissue. Eng., 2004,10(11-12):1716-24, doi: 10.1089/ten.2004.10.1716.
- 25.Zhang J.; Wilson G.F.; Soerens A.G.; et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res., 2009,104(4):e30-41, doi: 10.1161/CIRCRESAHA.108.192237.
- 26.Castañeda F.; Kinne R .K. Short exposure to millimolar concentrations of ethanol induces apoptotic cell death in multicellular HepG2 spheroids. J. Cancer. Res. Clin. Oncol., 2000,126(6):305-10, doi: 10.1007/s004320050348.
- 27.Bartholomä P.; Impidjati.; R.-M.A.; Zhang, Z.; et al. A More Aggressive Breast Cancer Spheroid Model Coupled to an Electronic Capillary Sensor System for a High-Content Screening of Cytotoxic Agents in Cancer Therapy: 3-Dimensional In Vitro Tumor Spheroids as a Screening Model. J. Biomol. Screen. 2005,10(7):705-14, doi: 10.1177/1087057105277841.
- 28.Niibe K.; Ohori-Morita Y.; Zhang M.; et al. A Shaking-Culture Method for Generating Bone Marrow Derived Mesenchymal Stromal/Stem Cell-Spheroids With Enhanced Multipotency in vitro. Front. Bioeng. Biotechnol., 2020; 8:590332, doi:10.3389/fbioe.2020.59033.
- 29.Carpenedo R.L.; Sargent C.Y.; McDevitt T.C. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem. Cells. 2007,25(9):2224-34, doi:10.1634/stemcells.2006-0523.
- 30.Qian X.; Nguyen H.N.; Song M.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016,165(5):1238-54, doi:10.1016/j.cell.2016.04.032 .
- 31.Achilli T.M., Meyer J.; Morgan J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther., 2012,12(10):1347-60, doi:10.1517/14712598.2012.707181.
- 32.Velasco V.; Shariati S.A.; Esfandyarpour R. Microtechnology-based methods for organoid models. Microsyst. Nanoeng., 2020,6(1):76, doi:10.1038/s41378-020-00185-3.
- 33.Li M.; Fu T.; Yang S.; et al. Agarose-based spheroid culture enhanced stemness and promoted odontogenic differentiation potential of human dental follicle cells in vitro. In Vitro Cell Dev. Biol. Anim., 2021,57(6):620-630. doi:10.1007/s11626-021-00591-5.
- 34.Cho M.O.; Li Z.; Shim H-E.; et al. Bioinspired tuning of glycol chitosan for 3D cell culture. NPG Asia Mater., 2016,8(9):e309-e, https://doi.org/10.1038/am.2016.130.
- 35.Rasouli R.; Tabrizian M. Rapid Formation of Multicellular Spheroids in Boundary-Driven Acoustic Microstreams. Small. 2021,17(39):2101931, https://doi.org/10.1002/smll.202101931.
- 36.Inubushi Y.; Tachibana A. Uniform spheroid formation on a laboratory-made, low cell attachment surface consisting of a chitin sheet. Biosci. Biotechnol. Biochem., 2020,84(5):997-1000, doi:10.1080/09168451.2020.1714423.
- 37.Carlsson J.; Yuhas J.M. Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res., 1984,95:1-23.
- 38.Mehta G.; Hsiao A.Y.; Ingram M.; et al. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control Release. 2012;164(2):192-204. doi:10.1016/j.jconrel.2012.04.045.
- 39.Alhaque S.; Themis M.; Rashidi H . Three-dimensional cell culture: from evolution to revolution. Philos. Trans. R. Soc. Lond. B Biol. Sci., 2018,373(1750):20170216. doi:10.1098/rstb.2017.0216.
- 40.Timmins N.E.; Nielsen L.K. Generation of Multicellular Tumor Spheroids by the Hanging-Drop Method. In: Hauser H, Fussenegger M, editors. Tissue Engineering. Totowa, NJ: Humana Press; 2007. p. 141-51.
- 41.Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp., 2011,(51), doi:10.3791/2720.
- 42.Jeong Y.; Tin A.; Irudayaraj J. Flipped Well-Plate Hanging-Drop Technique for Growing Three-Dimensional Tumors. Front. Bioeng. Biotechnol., 2022,10:898699. doi:10.3389/fbioe.2022.898699.
- 43.Rodoplu D.; Matahum J.S.; Hsu C.-H. A microfluidic hanging drop-based spheroid co-culture platform for probing tumor angiogenesis. Lab. Chip. 2022,22(7):1275-85, doi:10.1039/d1lc01177d.
- 44.Cho C.-Y.; Chiang T.-H.; Hsieh L.-H.; et al. Development of a Novel Hanging Drop Platform for Engineering Controllable 3D Microenvironments. Front. Cell Dev. Biol., 2020,8. doi:10.3389/fcell.2020.00327.
- 45.Grandhi T.S.P.; To J.; Romero A.; et al. High-throughput CRISPR-mediated 3D enrichment platform for functional interrogation of chemotherapeutic resistance. Biotechnol. Bioeng., 2021,118(8):3187-99, doi:10.1002/bit.27844.
- 46.Vinci M.; Gowan S.; Boxall F.; et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol., 2012,10:29, doi:10.1186/1741-7007-10-29.
- 47.Liao W.; Wang J.; Xu J.; et al. High-throughput three-dimensional spheroid tumor model using a novel stamp-like tool. J. Tissue Eng., 2019, 10:2041731419889184, doi:10.1177/2041731419889184.
- 48.Molyneaux K.; Wnek M.D.; Craig S.E.L.; et al. Physically-cross-linked poly(vinyl alcohol) cell culture plate coatings facilitate preservation of cell–cell interactions, spheroid formation, and stemness. J. Biomed. Mater. Res. B. Appl. Biomater.,. 2021, 109(11):1744-53, doi:10.1002/jbm.b.34832.
- 49.Otsuka H.; Sasaki K.; Okimura S.; et al. Micropatterned co-culture of hepatocyte spheroids layered on non-parenchymal cells to understand heterotypic cellular interactions. Sci. Technol. Adv. Mater., 2013, 14(6):065003, doi:10.1088/1468-6996/14/6/065003.
- 50.Valdoz J.C.; Jacobs D.J.; Cribbs C.G.; et al. An Improved Scalable Hydrogel Dish for Spheroid Culture. Life (Basel). 2021, 11(6), doi:10.3390/life11060517.
- 51.Lee J.M; Park D.Y.; Yang L.;et al. Generation of uniform-sized multicellular tumor spheroids using hydrogel microwells for advanced drug screening. Sci. Rep., 2018, 8(1):17145, doi:10.1038/s41598-018-35216-7.
- 52.Chao C.; Ngo L.P.; Engelward B.P. SpheroidChip: Patterned Agarose Microwell Compartments Harboring HepG2 Spheroids are Compatible with Genotoxicity Testing. ACS Biomater. Sci. Eng., 2020, 6(4):2427-39, doi:10.1021/acsbiomaterials.9b01951.
- 53.Ho V.H.B.; Müller K.H.; Barcza A.; et al. Generation and manipulation of magnetic multicellular spheroids. Biomaterials. 2010, 31(11):3095-102, doi:10.1016/j.biomaterials.2009.12.047.
- 54.Gaitán-Salvatella I.; López-Villegas E.O.; González-Alva P.; et al. Case Report: Formation of 3D Osteoblast Spheroid Under Magnetic Levitation for Bone Tissue Engineering. Front. Mol. Biosci., 2021, 8, doi:10.3389/fmolb.2021.672518.
- 55.Perez J.E.; Nagle I.; Wilhelm C. Magnetic molding of tumor spheroids: emerging model for cancer screening. Biofabrication. 2021, 13(1):015018, doi:10.1088/1758-5090/abc670.
- 56.Kim J.A. ; Choi J .-H.; Kim M.;et al. High-throughput generation of spheroids using magnetic nanoparticles for three-dimensional cell culture. Biomaterials. 2013, 34(34):8555-63, doi:10.1016/j.biomaterials.2013.07.056.
- 57.Fürsatz M.; Gerges P.; Wolbank S.; et al. Autonomous spheroid formation by culture plate compartmentation. Biofabrication. 2021, 13(3):035018, doi:10.1088/1758-5090/abe186.
- 58.Fürsatz M.; Gerges P.; Wolbank S.; et al. A novel system inducing autonomous spheroid formation of cell monolayers. Osteoarthritis and Cartilage. 2021, 29:S407-S8, doi:10.1016/j.joca.2021.02.530.
- 59.Gao M.; Harper M.M.; Lin M.; et al. Development of a Single-Cell Technique to Increase Yield and Use of Gastrointestinal Cancer Organoids for Personalized Medicine Application. J. Am. Coll. Surg., 2021, 232(4):504-14,doi:10.1016/j.jamcollsurg.2020.11.009.
- 60.Lee S.Y.; Teng Y.; Son M.; et al. High-dose drug heat map analysis for drug safety and efficacy in multi-spheroid brain normal cells and GBM patient-derived cells. PLoS One. 2021, 16(12):e0251998, doi:10.1371/journal.pone.0251998.
- 61.Vadivelu R.K.; Ooi C.H.; Yao R.-Q.;et al. Generation of three-dimensional multiple spheroid model of olfactory ensheathing cells using floating liquid marbles. Sci. Rep., 2015, 5(1):15083, doi:10.1038/srep15083.
- 62.Chen M.; Shah M.P.; Shelper T.B.; et al. Naked Liquid Marbles: A Robust Three-Dimensional Low-Volume Cell-Culturing System. ACS Appl. Mater. Interfaces. 2019, 11(10):9814-23, doi:10.1021/acsami.8b22036.
- 63.Schindler M.; Siriwardena D.; Kohler T.N.; et al. Agarose microgel culture delineates lumenogenesis in naive and primed human pluripotent stem cells. Stem Cell Reports. 2021, 16(5):1347-62, doi:10.1016/j.stemcr.2021.04.009.
- 64.Cui X.; Liu Y.; Hartanto Y.; et al. Multicellular Spheroids Formation and Recovery in Microfluidics-generated Thermoresponsive Microgel Droplets. Coll. Interf. Scie. Comm., 2016, 14:4-7.https://doi.org/10.1016/j.colcom.2016.09.001.
- 65.Chen D.; Tan Y.; Li Z.; et al. Organoid Cultures Derived From Patients With Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab., 2021, 106(5):1410-26, doi: 10.1210/clinem/dgab020.
- 66.Kim J.; Koo B.-K.; Knoblich J.A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol., 2020, 21(10):571-84, doi:10.1038/s41580-020-0259-3.
- 67.Sato T.; Vries R.G.; Snippert H.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009, 459(7244):262-5, doi:10.1038/nature07935.
- 68.Takahashi K.; Tanabe K.; Ohnuki M.; et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007, 131(5):861-72, doi:10.1016/j.cell.2007.11.019.
- 69.Spence J.R.; Mayhew C.N.; Rankin S.A.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011, 470(7332):105-9, doi:10.1038/nature09691.
- 70.Wallach T.E.; Bayrer J.R. Intestinal Organoids: New Frontiers in the Study of Intestinal Disease and Physiology. J. Pediatr. Gastroenterol. Nutr., 2017, 64(2):180-5, doi:10.1097/MPG.0000000000001411.
- 71.Crespo M.; Vilar E.; Tsai S.Y.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med., 2017, 23(7):878-84, doi:10.1038/nm.4355.
- 72.Rodansky E.S.; Johnson L.A.; Huang S.; et al Intestinal organoids: A model of intestinal fibrosis for evaluating anti-fibrotic drugs. Exp. Mol. Pathol., 2015, 98(3):346-51, doi:10.1016/j.yexmp.2015.03.033.
- 73.Li V.S.W. Modelling intestinal inflammation and infection using ‘mini-gut’ organoids. Nat. Rev. Gastroenterol. Hepatol., 2021, 18(2):89-90, doi:10.1038/s41575-020-00391-4.
- 74.van de Wetering M.; Francies H.E.; Francis J.M.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015, 161(4):933-45, doi:10.1016/j.cell.2015.03.053.
- 75.Yin Y.-B.; de Jonge H.R.; Wu X.;et al. Mini-gut: a promising model for drug development. Drug Disc. Today. 2019, 24(9):1784-94, doi:10.1016/j.drudis.2019.06.006.
- 76.Yoshida S., Miwa H.; Kawachi T.; et al. Generation of intestinal organoids derived from human pluripotent stem cells for drug testing. Sci. Rep., 2020, 10(1):5989, doi:10.1038/s41598-020-63151-z.
- 77.Vlachogiannis G.; Hedayat S.; Vatsiou A.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018, 359(6378):920-6, doi:10.1126/science.aao2774.
- 78.Norkin M.; Ordóñez-Morán P.; Huelsken J. High-content.; targeted RNA-seq screening in organoids for drug discovery in colorectal cancer. Cell Rep., 2021, 35(3):109026, doi:10.1016/j.celrep.2021.109026.
- 79.Raven A.; Lu W.-Y.; Man T.Y.;et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017, 547(7663):350-4, doi:10.1038/nature23015.
- 80.Huch M.; Dorrell C.; Boj S.F.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013, 494(7436):247-50, doi:10.1038/nature11826.
- 81.Huch M.; Gehart H.; Van Boxtel R.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015, 160(1-2):299-312, doi:10.1016/j.cell.2014.11.050.
- 82.Takebe T.; Sekine K.; Enomura M.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013, 499(7459):481-4, doi:10.1038/nature12271.
- 83.Guan Y.; Xu D., Garfin P.M.; et al. Human hepatic organoids for the analysis of human genetic diseases. JCI insight. 2017, 2(17), doi:10.1172/jci.insight.94954.
- 84.Vyas D.; Baptista P.M.; Brovold M.; et al. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology. 2018, 67(2):750-61, doi:10.1002/hep.29483.
- 85.Shinozawa T.; Kimura M.; Cai Y.; et al. High-Fidelity Drug-Induced Liver Injury Screen Using Human Pluripotent Stem Cell–Derived Organoids. Gastroenterology. 2021, 160(3):831-46.e10, doi:10.1053/j.gastro.2020.10.002.
- 86.Mun S.J.; Ryu J .-S.; Lee M-O.;et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol., 2019, 71(5):970-85, doi:10.1016/j.jhep.2019.06.030.
- 87.Shinozawa T.; Kimura M.; Cai Y.; et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell–derived organoids. Gastroenterology. 2021, 160(3):831-46. e10, doi:10.1053/j.gastro.2020.10.002.
- 88.Schwank G.; Koo B.-K.; Sasselli V.;et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem cell. 2013, 13(6):653-8, doi:10.1016/j.stem.2013.11.002.
- 89.Nie Y.-Z.; Zheng Y.-W.; Miyakawa K.; et al. Recapitulation of hepatitis B virus–host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine. 2018, 35:114-23, doi:10.1016/j.ebiom.2018.08.014.
- 90.Baktash Y.; Madhav A.; Coller K.E.; et al. Single particle imaging of polarized hepatoma organoids upon hepatitis C virus infection reveals an ordered and sequential entry process. Cell Host Microbe. 2018, 23(3):382-94. e5, doi:10.1016/j.chom.2018.02.005.
- 91.De Crignis E.; Hossain T.; Romal S.; et al. Application of human liver organoids as a patient-derived primary model for HBV infection and related hepatocellular carcinoma. eLife. 2021, 10:e60747, doi:10.7554/eLife.60747.
- 92.Nuciforo S.; Fofana I.; Matter M.S.; et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep., 2018, 24(5):1363-76, doi:10.1016/j.celrep.2018.07.001 .
- 93.Li L.; Knutsdottir H.; Hui K.; et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI insight. 2019, 4(2), doi:10.1172/jci.insight.121490.
- 94.Broutier L.; Mastrogiovanni G.; Verstegen M.; et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nat. Med., 2017, 23(12):1424-35, doi:10.1038/nm.4438.
- 95.Artegiani B.; van Voorthuijsen L.; Lindeboom R.G.; et al. Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell. 2019, 24(6):927-43. e6, doi:10.1016/j.stem.2019.04.017.
- 96.Khetani S.R. Pluripotent Stem Cell-Derived Human Liver Organoids Enter the Realm of High-Throughput Drug Screening. Gastroenterology. 2021, 160(3):653-5, doi:10.1053/j.gastro.2020.12.005 .
- 97.Shinozawa T.; Kimura M.; Cai Y.; et al. High-Fidelity Drug-Induced Liver Injury Screen Using Human Pluripotent Stem Cell-Derived Organoids. Gastroenterology. 2021, 160(3):831-46 e10, doi:10.1053/j.gastro.2020.10.002.
- 98.Huang L.; Holtzinger A.; Jagan I.; et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015, 21(11):1364-71, doi:10.1038/nm.3973.
- 99.Greggio C.; De Franceschi F.; Figueiredo-Larsen M.; et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013, 140(21):4452-62, doi:10.1242/dev.096628.
- 100.Grapin-Botton A. Three-dimensional pancreas organogenesis models. Diabetes Obes. Metab., 2016, 18(S1):33-40, doi:10.1111/dom.12720.
- 101.Nostro M.C.; Sarangi F.; Yang C.; et al. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Reports. 2015, 4(4):591-604, doi:10.1016/j.stemcr.2015.02.017.
- 102.Hohwieler M.; Illing A.; Hermann P.C.; et al. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut. 2017, 66(3):473-86, doi:10.1136/gutjnl-2016-312423.
- 103.Dahl-Jensen S.B.; Figueiredo-Larsen M.; Grapin-Botton A.; et al. Short-range growth inhibitory signals from the epithelium can drive non-stereotypic branching in the pancreas. Phys. Biol., 2016, 13(1):016007, doi:10.1088/1478-3975/13/1/016007.
- 104.Driehuis E.; Gracanin A.; Vries R.G.J.; Clevers H , Boj S.F. Establishment of Pancreatic Organoids from Normal Tissue and Tumors. STAR Protoc., 2020, 1(3):100192, doi:10.1016/j.xpro.2020.100192.
- 105.Tiriac H.; Belleau P.; Engle D.D.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov., 2018, 8(9):1112-29, doi:10.1158/2159-8290.CD-18-0349.
- 106.Hirt C.K.; Booij T.H.; Grob L.; et al. Drug screening and genome editing in human pancreatic cancer organoids identifies drug-gene interactions and candidates for off-label therapy. Cell Genomics. 2022, 2(2):100095, doi:10.1016/j.xgen.2022.100095.
- 107.Hou S.; Tiriac H.; Sridharan B.P.; et al. Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening. SLAS DISC:, 2018, 23(6):574-84.doi:10.1177/2472555218766842.
- 108.Shi X.; Li Y.; Yuan Q.; et al. Integrated profiling of human pancreatic cancer organoids reveals chromatin accessibility features associated with drug sensitivity. Nat. Commu., 2022, 13(1):2169.doi:10.1038/s41467-022-29857-6.
- 109.Yin J.; Meng H.; Lin J.; et al. Pancreatic islet organoids-on-a-chip: how far have we gone? J. Nanobiotechnology. 2022, 20(1):308, doi:10.1186/s12951-022-01518-2.
- 110.Lancaster M.A.; Renner M.; Martin C.-A.;et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013, 501(7467):373-9, doi:10.1038/nature12517.
- 111.Cho A.-N.; Jin Y.; An Y.;et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun., 2021, 12(1):4730, doi:10.1038/s41467-021-24775-5.
- 112.Lee C.-T.; Bendriem R.M.; Wu W.W.; et al. 3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders. Journal of Biomedical Science. 2017, 24(1):59, doi:10.1186/s12929-017-0362-8.
- 113.Ao Z.; Cai H.; Wu Z.; et al . Controllable fusion of human brain organoids using acoustofluidics. Lab Chip. 2021, 21(4):688-99, doi:10.1039/d0lc01141j.
- 114.Di Lullo E.; Kriegstein A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci., 2017, 18(10):573-84, doi:10.1038/nrn.2017.107.
- 115.Luo J. ; Li P . Human pluripotent stem cell-derived brain organoids as in vitro models for studying neural disorders and cancer. Cell Bios., 2021, 11(1):99, doi:10.1186/s13578-021-00617-1.
- 116.Qian X.; Nguyen H.N.; Jacob F.; et al. Using brain organoids to understand Zika virus-induced microcephaly. Development. 2017, 144(6):952-7, doi:10.1242/dev.140707.
- 117.Gabriel E.; Ramani A.; Karow U.; et al. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell. 2017, 20(3):397-406.e5, doi:10.1016/j.stem.2016.12.005.
- 118.Lee S.-E.; Shin N.; Kook M.G.;et al. Human iNSC-derived brain organoid model of lysosomal storage disorder in Niemann–Pick disease type C. Cell Death Dis., 2020, 11(12):1059.doi:10.1038/s41419-020-03262-7.
- 119.Zhang L.; Liu F.; Weygant N.; et al. A novel integrated system using patient-derived glioma cerebral organoids and xenografts for disease modeling and drug screening. Cancer Lett., 2021, 500:87-97.doi:10.1016/j.canlet.2020.12.013.
- 120.Goranci-Buzhala G.; Mariappan A.; Gabriel E.; et al. Rapid and Efficient Invasion Assay of Glioblastoma in Human Brain Organoids. Cell Rep., 2020, 31(10):107738, doi:10.1016/j.celrep.2020.107738.
- 121.Mansour A.A.; Gonçalves J.T.; Bloyd C.W.; et al. An in vivo model of functional and vascularized human brain organoids. Nature Biotechnology. 2018, 36(5):432-41, doi:10.1038/nbt.4127.
- 122.Tran H .N; Gautam V. Micro- and nanodevices for integration with human brain organoids. Biosens. Bioelectro., 2022, 114734. doi:1016/j.bios.2022.114734
- 123.Fischer J.; Heide M.; Huttner W.B. Genetic Modification of Brain Organoids. Front. Cell. Neurosci., 2019, 13, doi:10.3389/fncel.2019.00558.
- 124.Durens M.; Nestor J.; Williams M.; et al. High-throughput screening of human induced pluripotent stem cell-derived brain organoids. Journal of Neuroscience Methods. 2020, 335:108627.
- 125.Park J.-C.; Jang S.-Y.; Lee D.; et al. A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids. Nat. Commu., 2021, 12(1):280.doi:10.1038/s41467-020-20440-5.
- 126.Nestor M.W.; Paull D.; Jacob S.; et al. Differentiation of serum-free embryoid bodies from human induced pluripotent stem cells into networks. Stem Cell Res. 2013, 10(3):454-63.doi:10.1016/j.scr.2013.02.001.
- 127.Brennand K.J.; Marchetto M.C.; Benvenisty N.; et al. Creating Patient-Specific Neural Cells for the In Vitro Study of Brain Disorders. Stem Cell Reports. 2015, 5(6):933-45, doi:10.1016/j.stemcr.2015.10.011.
- 128.Nugraha B.; Buono M.F.; von Boehmer L.; et al. Human Cardiac Organoids for Disease Modeling. Clin. Pharmacol. Ther., 2019, 105(1):79-85.doi:10.1002/cpt.1286.
- 129.Beauchamp P.; Jackson C.B.; Ozhathil L.C.; et al. 3D Co-culture of hiPSC-Derived Cardiomyocytes With Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids. Front. Mol. Biosci., 2020, 7.doi:10.3389/fmolb.2020.00014.
- 130.Filippo Buono M.; von Boehmer L.; Strang J.; et al. Human Cardiac Organoids for Modeling Genetic Cardiomyopathy. Cells. 2020, 9(7):1733, doi:10.3390/cells9071733.
- 131.Richards D.J.; Li Y.; Kerr C.M.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng., 2020, 4(4):446-62, doi:10.1038/s41551-020-0539-4.
- 132.Skardal A.; Shupe T.; Atala A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today. 2016, 21(9):1399-411.doi:10.1016/j.drudis.2016.07.003.
- 133.Forsythe S.D.; Devarasetty M.; Shupe T.; et al. Environmental Toxin Screening Using Human-Derived 3D Bioengineered Liver and Cardiac Organoids. Front. Public. Health. 2018, 6, doi:10.3389/fpubh.2018.00103.
- 134.Hoang P.; Kowalczewski A.; Sun S.; et al. Engineering spatial-organized cardiac organoids for developmental toxicity testing. Stem Cell Reports. 2021, 16(5):1228-44, doi:10.1016/j.stemcr.2021.03.013.
- 135.Mills R.J.; Titmarsh D.M.; Koenig X.; et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A. 2017, 114(40):E8372-E81, doi:10.1073/pnas.1707316114.
- 136.Zhang Y.S.; Arneri A.; Bersini S.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016, 110:45-59.doi:10.1016/j.biomaterials.2016.09.003.
- 137.Cho J.; Lee H.; Rah W.; et al. From engineered heart tissue to cardiac organoid. Theranostics. 2022, 12(6):2758-72.doi:10.7150/thno.67661.
- 138.Wimmer R.A.; Leopoldi A.; Aichinger M.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019, 565(7740):505-10, doi:10.1038/s41586-018-0858-8.
- 139.Kim H.; Kamm R.D.; Vunjak-Novakovic G.; et al. Progress in multicellular human cardiac organoids for clinical applications. Cell Stem Cell. 2022, 29(4):503-14, doi:10.1016/j.stem.2022.03.012.
- 140.Drakhlis L.; Biswanath S.; Farr C.-M.;et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol., 2021, 39(6):737-46, doi:10.1038/s41587-021-00815-9.
- 141.Drakhlis L.; Devadas S.B.; Zweigerdt R. Generation of heart-forming organoids from human pluripotent stem cells. Nat. Protoc., 2021, 16(12):5652-72, doi:10.1038/s41596-021-00629-8.
- 142.Lewis-Israeli Y.R.; Wasserman A.H.; Gabalski M.A.; et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat Commun. 2021, 12(1):5142, doi:10.1038/s41467-021-25329-5.
- 143.Hofbauer P.; Jahnel S.M.; Papai N.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell. 2021, 184(12):3299-317 e22, doi:10.1016/j.cell.2021.04.034 .
- 144.Lee J.; Sutani A.; Kaneko R. ; et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat. Commu., 2020, 11(1):4283.doi:10.1038/s41467-020-18031-5.
- 145.Rossi G.; Broguiere N.; Miyamoto M.; et al. Capturing Cardiogenesis in Gastruloids. Cell Stem Cell. 2021, 28(2):230-40 e6, doi:10.1016/j.stem.2020.10.013 .
- 146.Lewis-Israeli Y.R.; Abdelhamid M. ; Olomu I.; et al. Modeling the Effects of Maternal Diabetes on the Developing Human Heart Using Pluripotent Stem Cell–Derived Heart Organoids. Curr. Protoc., 2022, 2(6):e461, doi:10.1002/cpz1.461.
- 147.Skardal A.; Aleman J.; Forsythe S.; et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication. 2020, 12(2):025017, doi:10.1088/1758-5090/ab6d36.
- 148.Park S.E.; Georgescu A.; Huh D. Organoids-on-a-chip. Science. 2019, 364(6444):960-5, doi:10.1126/science.aaw7894.
- 149.Ferrari E.; Rasponi M. Liver–Heart on chip models for drug safety. APL Bioengineering. 2021, 5(3):031505, doi:10.1063/5.0048986.
- 150.Parlato S.; Grisanti G.; Sinibaldi G.; et al. Tumor-on-a-chip platforms to study cancer–immune system crosstalk in the era of immunotherapy. Lab Chip. 2021, 21(2):234-53, doi:10.1039/d0lc00799d.
How to Cite
Liu, Y.; Xu, H.; Abraham, S.; Wang, X.; Keavney, B. D. Progress of 3D Organoid Technology for Preclinical Investigations: Towards Human In Vitro Models. International Journal of Drug Discovery and Pharmacology 2022, 1 (1), 9. https://doi.org/10.53941/ijddp.v1i1.188.
RIS
BibTex
Copyright & License

Yingjuan Liu, Honglin Xu
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


