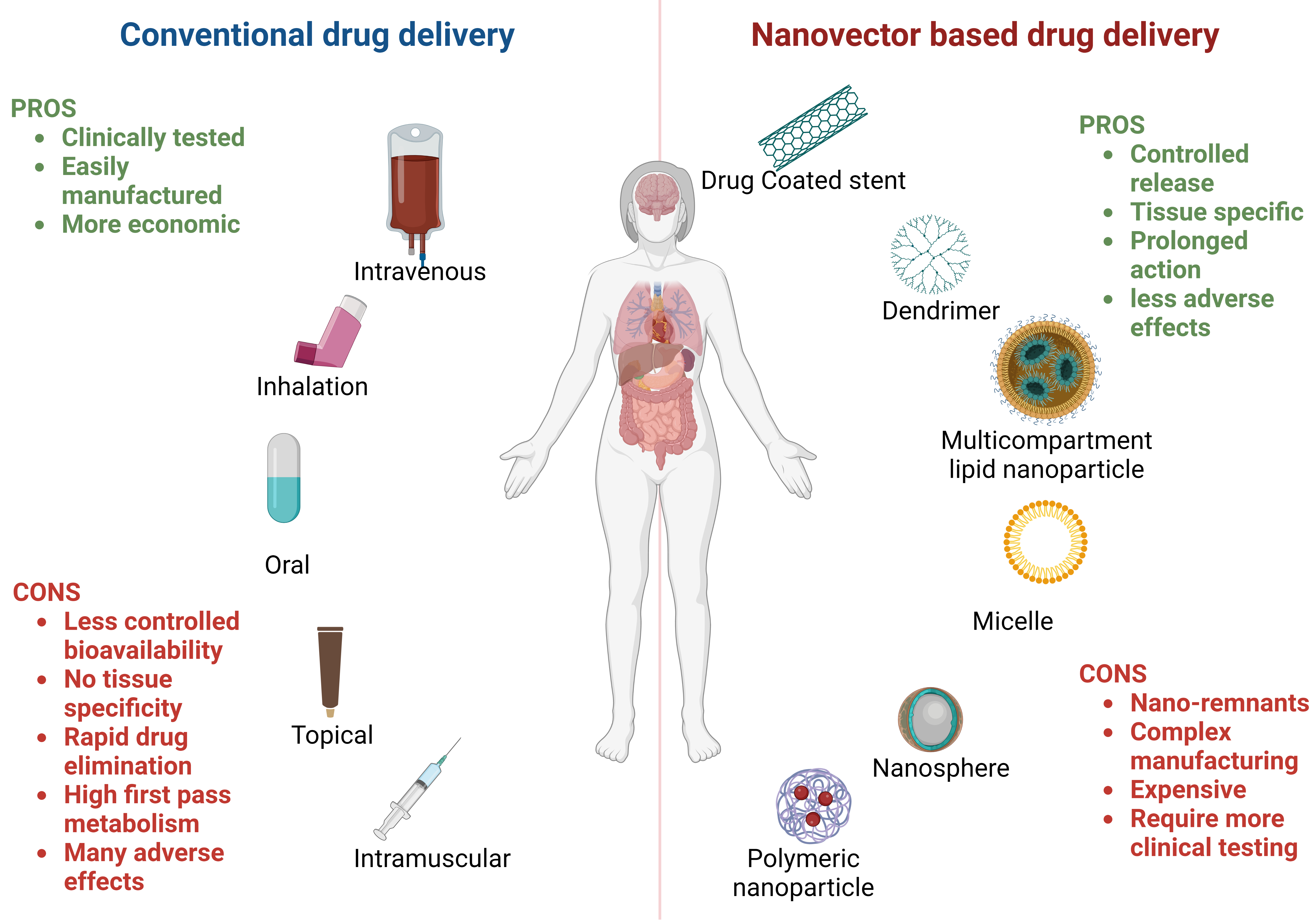
The conventional drug delivery systems have several limitations, such as the high frequency of administration, several off-target effects, and the need for tissue specificity. Recently, smart drug shuttles have emerged, and the nano applications provided a new opportunity for advancing the drug delivery system to become tissue targeted and decrease the frequency of administration. The recent development of nanovectors as drug carriers has gone through several steps of evolution that ended with the development of logic-embedded nanovectors. Here, we summarize the different types of nanovectors and their applications in various clinical situations, and finally, we spot the light on the future of this area of research.
- Open Access
- Review
Controlled and Targeted Drug Delivery Using Smart Nanovectors
- Abou Bakr M. Salama 1, 2,
- Yasmin Y. Salem 1, 2,
- Tamer M. A. Mohamed 1, 3, 4, 5, 6, *
Author Information
Received: 28 Jan 2023 | Accepted: 27 Feb 2023 | Published: 20 Mar 2023
Abstract
Graphical Abstract
Keywords
References
- 1.Canal P.; Gamelin E.; Vassal G.; et al. Benefits of pharmacological knowledge in the design and monitoring of cancer chemotherapy. Pathol. Oncol. Res., 1998, 4(3): 171-178.
- 2.Caliceti P.; Veronese F.M. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)–protein conjugates. Adv. Drug Delivery Rev., 2003, 55(10): 1261-1277.
- 3.Moghimi S.M.; Davis S.S. Innovations in avoiding particle clearance from blood by Kupffer cells: cause for reflection. Crit. Rev. Ther. Drug Carrier Syst., 1994, 11(1): 31-59.
- 4.Accardo A.; Aloj L.; Aurilio M.; et al. Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs. Int. J. Nanomed., 2014, 9: 1537-1557.
- 5.Mendes R.; Fernandes A.R.; Baptista P.V. Gold nanoparticle approach to the selective delivery of gene silencing in cancer—the case for combined delivery? Genes, 2017, 8(3): 94.
- 6.Permana A.D.; Anjani Q.K.; Sartini.; et al. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater. Sci. Eng.: C , 2021, 120: 111786.
- 7.Shi H.D.; Liu S.Z.; Cheng J.J.; et al. Charge-selective delivery of proteins using mesoporous silica nanoparticles fused with lipid bilayers. ACS Appl. Mater. Interfaces, 2019, 11(4): 3645-3653.
- 8.Oake A.; Bhatt P.; Pathak V.V. Understanding surface characteristics of nanoparticles. Pathak Y.V. Surface modification of nanoparticles for targeted drug delivery. Cham: Springer, 2019: 1-17.
- 9.Duan X.P.; Li Y.P. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small, 2013, 9(9/10): 1521-1532.
- 10.Jones M.; Leroux J. Polymeric micelles - a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm., 1999, 48(2): 101-111.
- 11.Nishiyama N.; Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther., 2006, 112(3): 630-648.
- 12.Aghebati-Maleki A.; Dolati S.; Ahmadi M.; et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol., 2020, 235(3): 1962-1972.
- 13.Gavas S.; Quazi S.; Karpiński T.M. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res. Lett., 2021, 16(1): 173.
- 14.Alshawwa S.Z.; Kassem A.A.; Farid R.M.; et al. Nanocarrier drug delivery systems: characterization, limitations, future perspectives and implementation of artificial intelligence. Pharmaceutics, 2022, 14(4): 883.
- 15.Bhatia S. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. Bhatia S. Natural polymer drug delivery systems. Cham: Springer, 2016: 33-93.
- 16.Patra J.K.; Das G.; Fraceto L.F.; et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol., 2018, 16(1): 71.
- 17.Aithal A.; Aithal P.S. The concept of ideal drug & its realization opportunity using nano pharmaceutical research scenario. International Journal of Health Sciences and Pharmacy, 2018, 2(2): 11-26.
- 18.LaVan D.A.; McGuire T.; Langer R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol., 2003, 21(10): 1184-1191.
- 19.Ruiz M.E.; Scioli Montoto S. Routes of drug administration. Talevi A.; Quiroga P.A.M. ADME processes in pharmaceutical sciences: dosage, design, and pharmacotherapy success. Cham: Springer, 2018: 97-133.
- 20.Allen T.M.; Cullis P.R. Drug delivery systems: entering the mainstream. Science, 2004, 303(5665): 1818-1822.
- 21.Zunhammer M.; Ploner M.; Engelbrecht C.; et al. The effects of treatment failure generalize across different routes of drug administration. Sci. Transl. Med., 2017, 9(393): eaal2999.
- 22.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. department of health and human services, Centers for Disease Control and Prevention, 2011.
- 23.Kadian R.; Nanda A. A comprehensive insight on recent advancements in self-emulsifying drug delivery systems. Curr. Drug Delivery. 2022, in press.
- 24.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer, 2005, 5(3): 161-171.
- 25.Amiji M.M. Nanotechnology for cancer therapy. Boca Raton: CRC Press, 2006.
- 26.Riehemann K.; Schneider S.W.; Luger T.A.; et al. Nanomedicine--challenge and perspectives. Angew. Chem., Int. Ed. Engl., 2009, 48(5): 872-897.
- 27.Hashizume H.; Baluk P.; Morikawa S.; et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol., 2000, 156(4): 1363-1380.
- 28.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul., 2001, 41: 189-207.
- 29.Torchilin V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discovery, 2005, 4(2): 145-160.
- 30.Hari S.K.; Gauba A.; Shrivastava N.; et al. Polymeric micelles and cancer therapy: an ingenious multimodal tumor-targeted drug delivery system. Drug Delivery Transl. Res., 2023, 13(1): 135-163.
- 31.Brannon-Peppas L.; Blanchette J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Delivery Rev., 2004, 56(11): 1649-1659.
- 32.Kale A.A.; Torchilin V.P. “Smart” drug carriers: PEGylated TATp-modified pH-sensitive liposomes. J. Liposome Res., 2007, 17(3/4): 197-203.
- 33.Farokhzad O.C.; Langer R. Impact of nanotechnology on drug delivery. ACS Nano, 2009, 3(1): 16-20.
- 34.Souza G.R.; Staquicini F.I.; Christianson D.R.; et al. Combinatorial targeting and nanotechnology applications. Biomed. Microdevices, 2010, 12(4): 597-606.
- 35.Juweid M.; Neumann R.; Paik C.; et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res., 1992, 52(19): 5144-5153.
- 36.Serda R.E.; Godin B.; Blanco E.; et al. Multi-stage delivery nano-particle systems for therapeutic applications. Biochim. Biophys. Acta, 2011, 1810(3): 317-329.
- 37.Godin B.; Serda R.E.; Liu X.W.; et al. Injectable multistage nanovectors for enhancing imaging contrast and directed therapy. Svenson, S.; Prud’homme, R.K. Multifunctional nanoparticles for drug delivery applications: nanostructure science and technology. Boston, MA: Springer, 2012: 201-223.
- 38.Souza G.R.; Christianson D.R.; Staquicini F.I.; et al. Networks of gold nanoparticles and bacteriophage as biological sensors and cell-targeting agents. Proc. Natl. Acad. Sci., 2006, 103(5): 1215-1220.
- 39.Sengupta S.; Eavarone D.; Capila I.; et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature, 2005, 436(7050): 568-572.
- 40.Chen A.M.; Zhang M.; Wei D.G.; et al. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small, 2009, 5(23): 2673-2677.
- 41.Tasciotti E.; Liu X.W.; Bhavane R.; et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat. Nanotechnol., 2008, 3(3): 151-157.
- 42.Khemtong C.; Kessinger C.W.; Ren J.M.; et al. In vivo off-resonance saturation magnetic resonance imaging of αvβ3-targeted superparamagnetic nanoparticles. Cancer Res., 2009, 69(4): 1651-1658.
- 43.Yoo H.S.; Park T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J. Controlled Release, 2004, 100(2): 247-256.
- 44.Jeong Y.I.; Seo S.J.; Park I.K.; et al. Cellular recognition of paclitaxel-loaded polymeric nanoparticles composed of poly(γ-benzyl l-glutamate) and poly(ethylene glycol) diblock copolymer endcapped with galactose moiety. Int. J. Pharm., 2005, 296(1/2): 151-161.
- 45.Carpin L.B.; Bickford L.R.; Agollah G.; et al. Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells. Breast Cancer Res. Treat., 2011, 125(1): 27-34.
- 46.Stoltenburg R.; Reinemann C.; Strehlitz B. SELEX—A (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng., 2007, 24(4): 381-403.
- 47.Thiviyanathan V.; Somasunderam A.D.; Gorenstein D.G. Combinatorial selection and delivery of thioaptamers. Biochem. Soc. Trans., 2007, 35(1): 50-52.
- 48.Röthlisberger P.; Hollenstein M. Aptamer chemistry. Adv. Drug Delivery Rev., 2018, 134: 3-21.
- 49.Esawi E.; Nsairat H.; Mahmoud I.S.; et al. 20 - Clinical use and future perspective of aptamers. Kesharwani P. Aptamers engineered nanocarriers for cancer therapy. Cambridge: Woodhead Publishing, 2023: 481-520.
- 50.Bai X.; Smith Z.L.; Wang Y.H.; et al. Sustained drug release from smart nanoparticles in cancer therapy: a comprehensive review. Micromachines, 2022, 13(10): 1623.
- 51.Bagherifam S.; Skjeldal F.M.; Griffiths G.; et al. pH-responsive nano carriers for doxorubicin delivery. Pharm. Res., 2015, 32(4): 1249-1263.
- 52.Yu C.; Wang L.; Xu Z.Z.; et al. Smart micelles self-assembled from four-arm star polymers as potential drug carriers for pH-triggered DOX release. J. Polym. Res., 2020, 27(5): 111.
- 53.Bagheri M.; Shateri S.; Niknejad H.; et al. Thermosensitive biotinylated hydroxypropyl cellulose-based polymer micelles as a nano-carrier for cancer-targeted drug delivery. J. Polym. Res., 2014, 21(10): 567.
- 54.Wang J.J. Combination treatment of cervical cancer using folate-decorated, pH-sensitive, carboplatin and paclitaxel co-loaded lipid-polymer hybrid nanoparticles. Drug Des., Dev. Ther., 2020, 14: 823-832.
- 55.Guo S.J.; Vieweger M.; Zhang K.M.; et al. Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nat. Commun., 2020, 11(1): 972.
- 56.Patil R.; Portilla-Arias J.; Ding H.; et al. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(β-L-malic acid). Pharm. Res., 2010, 27(11): 2317-2329.
- 57.Wang R.B.; Billone P.S.; Mullett W.M. Nanomedicine in action: an overview of cancer nanomedicine on the market and in clinical trials. J. Nanomater., 2013, 2013: 629681.
- 58.Saif M.W. U.S. food and drug administration approves paclitaxel protein-bound particles (abraxane®) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP. Journal of the Pancreas, 2013, 14(6): 686-688.
- 59.Martis E.; Badve R.; Degwekar M. Nanotechnology based devices and applications in medicine: an overview. Chron. Young Sci., 2012, 3(1): 68.
- 60.Anselmo A.C.; Mitragotri S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med., 2019, 4(3): e10143.
- 61.Pillai G. Nanomedicines for cancer therapy: an update of FDA approved and those under various stages of development. SOJ Pharmacy & Pharmaceutical Sciences, 2014, 1(2): 13.
- 62.Sharma G.; Sharma A.R.; Lee S.S.; et al. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm., 2019, 559: 360-372.
- 63.Deo M.R.; Sant V.P.; Parekh S.R.; et al. Proliposome-based transdermal delivery of levonorgestrel. J. Biomater. Appl., 1997, 12(1): 77-88.
- 64.Li J.; Zhang Z.X.; Zhang B.L.; et al. Transferrin receptor 1 targeted nanomedicine for brain tumor therapy. Biomater. Sci., 2023, in press.
- 65.Peters D.; Kastantin M.; Kotamraju V.R.; et al. Targeting atherosclerosis by using modular, multifunctional micelles. Proc. Natl. Acad. Sci., 2009, 106(24): 9815-9819.
- 66.Eniola-Adefeso O.; Heslinga M.J.; Porter T.M. Design of nano vectors for therapy and imaging of cardiovascular diseases. Methodist DeBakey Heart & Vascular Center, 2012, 8(1): 13-17.
- 67.Aikawa M.; Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc. Pathol., 2004, 13(3): 125-138.
- 68.Tölli M.A.; Ferreira M.P.A.; Kinnunen S.M.; et al. In vivo biocompatibility of porous silicon biomaterials for drug delivery to the heart. Biomaterials, 2014, 35(29): 8394-8405.
- 69.Pinchuk L.; Wilson G.J.; Barry J.J.; et al. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials, 2008, 29(4): 448-460.
- 70.Tzafriri A.R.; Edelman E.R. Endovascular drug delivery and drug elution systems: first principles. Interventional Cardiol. Clin., 2016, 5(3): 307-320.
- 71.Karanasiou G.S.; Papafaklis M.I.; Conway C.; et al. Stents: biomechanics, biomaterials, and insights from computational modeling. Ann. Biomed. Eng., 2017, 45(4): 853-872.
- 72.Kleemann E.; Schmehl T.; Gessler T.; et al. Iloprost-containing liposomes for aerosol application in pulmonary arterial hypertension: formulation aspects and stability. Pharm. Res., 2007, 24(2): 277-287.
- 73.Kan P.; Chen K.J.; Hsu C.F.; et al. Inhaled liposomal iloprost shows high drug encapsulation, extended release profile and potentials of improving patient compliance. Eur. Respir. J., 2018, 52: PA3038.
- 74.Rn K.; Gokhale P.C.; Kshirsagar N.A.; et al. Optimizing dosage regimens of liposomal amphotericin B using Aspergillus murine model. Indian J. Pharmacol., 1996, 28: 88.
- 75.Kshirsagar N.A.; Pandya S.K.; Kirodian G.B.; et al. Liposomal drug delivery system from laboratory to clinic. J. Postgrad. Med., 2005, 51(5): 5-15.
- 76.Tiwari G.; Tiwari R.; Sriwastawa B.; et al. Drug delivery systems: an updated review. Int. J. Pharm. Invest., 2012, 2(1): 2-11.
- 77.Sperry P.J.; Cua D.J.; Wetzel S.A.; et al. Antimicrobial activity of AmBisome and non-liposomal amphotericin B following uptake of Candida glabrata by murine epidermal Langerhans cells. Med. Mycol., 1998, 36(3): 135-141.
- 78.Zhu Y.X.; Che L.; He H.M.; et al. Highly efficient nanomedicines assembled via polymer-drug multiple interactions: tissue-selective delivery carriers. J. Controlled Release, 2011, 152(2): 317-324.
- 79.Bummer P.M. Physical chemical considerations of lipid-based oral drug delivery—solid lipid nanoparticles. Critical Reviews™ in Therapeutic Drug Carrier Systems, 2004, 21(1): 1-20.
- 80.Puoci F.; Iemma F.; Muzzalupo R.; et al. Spherical molecularly imprinted polymers (SMIPs) via a novel precipitation polymerization in the controlled delivery of sulfasalazine. Macromol. Biosci., 2004, 4(1): 22-26.
- 81.Priyam A.; Shivhare K.; Yadav S.; et al. Enhanced solubility and self-assembly of amphiphilic sulfasalazine-PEG-OMe (S-PEG) conjugate into core-shell nanostructures useful for colonic drug delivery. Colloids Surf., A, 2018, 547: 157-167.
- 82.Dhaneshwar S.S.; Gairola N.; Kandpal M.; et al. Synthesis, kinetic studies and pharmacological evaluation of mutual azo prodrugs of 5-aminosalicylic acid for colon-specific drug delivery in inflammatory bowel disease. Eur. J. Med. Chem., 2009, 44(10): 3922-3929.
- 83.Li J.H.; Zhang Z.Z.; Li J.; et al. Copper-olsalazine metal-organic frameworks as a nanocatalyst and epigenetic modulator for efficient inhibition of colorectal cancer growth and metastasis. Acta Biomater., 2022, 152: 495-506.
- 84.Levine D.J. Runčevski T.; Kapelewski M.T.;et al. Olsalazine-based metal-organic frameworks as biocompatible platforms for H2 adsorption and drug delivery. J. Am. Chem. Soc., 2016, 138(32): 10143-10150.
- 85.Hakkou K.; Molina-Pinilla I.; Rangel-Núñez C.; et al. Synthesis of novel (bio) degradable linear azo polymers conjugated with olsalazine. Polym. Degrad. Stab., 2019, 167: 302-312.
- 86.Cortez-Maya S.; Pedro-Hernández L.D.; Martínez-Klimova E.; et al. Anticancer activity of water-soluble olsalazine-PAMAM-dendrimer-salicylic acid-conjugates. Biomolecules, 2019, 9(8): 360.
- 87.Mukhtar M.; Fényes E.; Bartos C.; et al. Chitosan biopolymer, its derivatives and potential applications in nano-therapeutics: a comprehensive review. Eur. Polym. J., 2021, 160: 110767.
- 88.Chaudhury A.; Das S. Recent advancement of chitosan-based nanoparticles for oral controlled delivery of insulin and other therapeutic agents. AAPS PharmSciTech, 2011, 12(1): 10-20.
- 89.Sonia T.A.; Sharma C.P. An overview of natural polymers for oral insulin delivery. Drug Discovery Today, 2012, 17(13/14): 784-792.
- 90.Sarmento B.; Ribeiro A.; Veiga F.; et al. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res., 2007, 24(12): 2198-2206.
- 91.Chiang W.L.; Ke C.J.; Liao Z.X.; et al. Pulsatile drug release from PLGA hollow microspheres by controlling the permeability of their walls with a magnetic field. Small, 2012, 8(23): 3584-3588.
How to Cite
Salama, A. B. M.; Salem, Y. Y.; Mohamed, T. M. A. Controlled and Targeted Drug Delivery Using Smart Nanovectors. International Journal of Drug Discovery and Pharmacology 2023, 2 (1), 18–25. https://doi.org/10.53941/ijddp.0201010.
RIS
BibTex
Copyright & License

Abou Bakr Salama, Yasmin Salem, Tamer Mohamed
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


