Duchenne muscular dystrophy is caused by inadequate generation of functional dystrophin protein. Traditional clinical treatments can only slightly mitigate the progression of the disease, but not completely stem or reverse the decline in muscle function. Therapies aimed at dystrophin recovery are currently under development, among which are exon skipping and stop codon readthrough therapies. They are now used in clinics, while gene addition therapies are in phase III clinical trials. Gene editing therapies have also been approved for the first clinical trial recently. This review will discuss these emerging therapies, clinical trials, and directions for future developments.
- Open Access
- Review
Promising Treatments for Duchenne Muscular Dystrophy: Restoring Dystrophin Protein Expression Using Nucleic Acid Therapeutics
- Guo Hu,
- Chen Chen *
Author Information
Received: 10 Oct 2022 | Accepted: 10 Nov 2022 | Published: 11 Jan 2023
Abstract
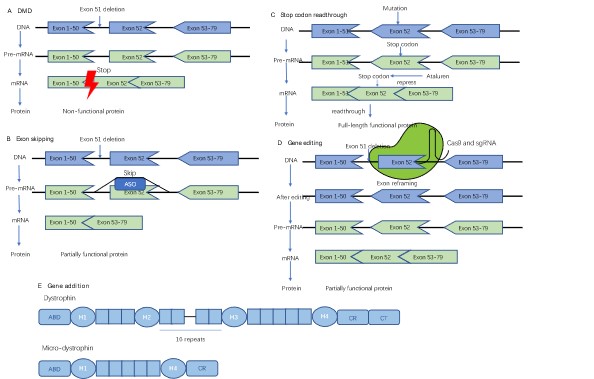
Graphical Abstract
References
- 1.Crisafulli S.; Sultana J.; Fontana A.; et al. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet. J. Rare Dis., 2020, 15(1): 141.
- 2.Mercuri E.; Bönnemann C.G.; Muntoni F. Muscular dystrophies. Lancet, 2019, 394(10213): 2025-38.
- 3.Zhao J.; Kodippili K.; Yue Y.; et al. Dystrophin contains multiple independent membrane-binding domains. Hum Mol Genet., 2016, 25(17): 3647-53.
- 4.Gao Q.Q.; McNally E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol., 2015, 5(3): 1223-39.
- 5.Monaco A.P.; Bertelson CJ; Liechti-Gallati S; et.al. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics, 1988, 2(1): 90-5.
- 6.Gloss D.; Moxley R.T.; Ashwal S.; et.al. Practice guideline update summary: Corticosteroid treatment of Duchenne muscular dystrophy: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology, 2016, 86(5): 465-72.
- 7.Verhaart I.E.C.; Aartsma-Rus A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol.,2019, 15(7): 373-86.
- 8.Aartsma-Rus A.; Van Deutekom J.C.T.; Fokkema I.F.; et.al. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve, 2006, 34(2): 135-44.
- 9.Bladen C.L.; Salgado D.; Monges S.; et al. The TREAT-NMD DMD Global Database: analysis of more than 7;000 Duchenne muscular dystrophy mutations. Hum. Mutat., 2015, 36(4): 395-402.
- 10.Duan D.; Goemans N.; Takeda S.; et.al. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers., 2021, 7(1): 13.
- 11.McDonald C.M.; Shieh P.B.; Abdel-Hamid H.Z.; et al. Open-Label Evaluation of Eteplirsen in Patients with Duchenne Muscular Dystrophy Amenable to Exon 51 Skipping: PROMOVI Trial. J. Neuromuscul. Dis., 2021, 8(6).
- 12.Mendell J.R.; Rodino-Klapac L.R.; Sahenk Z.; et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol., 2013, 74(5): 637-47.
- 13.Mattingly T.J.; Simoni-Wastila L. Patient-Centered Drug Approval: The Role of Patient Advocacy in the Drug Approval Process. J. Manag. Care Spec. Pharm., 2017, 23(10): 1078-82.
- 14.Jirka S.M.G.; t Hoen P.A.C.; Diaz Parillas V.; et al. Cyclic Peptides to Improve Delivery and Exon Skipping of Antisense Oligonucleotides in a Mouse Model for Duchenne Muscular Dystrophy. Mol. Ther., 2018, 26(1): 132-47.
- 15.Sarepta Therapeutics I. Sarepta Therapeutics Announces Positive Clinical Results from MOMENTUM, a Phase 2 Clinical Trial of SRP-5051 in Patients with Duchenne Muscular Dystrophy Amenable to Skipping Exon 51. (accessed Oct 1,2022). https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-positive-clinical-results.
- 16.Sarepta Therapeutics I. Sarepta Therapeutics Reports Positive Clinical Results from Phase 2 MOMENTUM Study of SRP-5051 in Patients with Duchenne Muscular Dystrophy Amenable to Skipping Exon 51. (accessed Oct 1,2022). https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-reports-positive-clinical-results-phase-2.
- 17.Sarepta Therapeutics I. Sarepta Therapeutics Provides Update on SRP-5051 for the Treatment of Duchenne Muscular Dystrophy. (accessed Oct 1,2022). https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-provides-update-srp-5051-treatment-duchenne.
- 18.Sarepta Therapeutics I. Sarepta Therapeutics Announces That FDA has Lifted its Clinical Hold on SRP-5051 for the Treatment of Duchenne Muscular Dystrophy. (accessed Oct 1,2022). https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-fda-has-lifted-its-clinical-hold.
- 19.Lee J.; Echigoya Y.; Duddy W.; et al. Antisense PMO cocktails effectively skip dystrophin exons 45-55 in myotubes transdifferentiated from DMD patient fibroblasts. PLoS One., 2018, 13(5): e0197084.
- 20.Ng M.Y.; Li H.; Ghelfi M.D., et al.. Ataluren and aminoglycosides stimulate read-through of nonsense codons by orthogonal mechanisms. Proc. Natl. Acad. Sci. USA, 2021, 118(2).
- 21.Huang S.; Bhattacharya A.; Ghelfi M.D.; et al. Ataluren binds to multiple protein synthesis apparatus sites and competitively inhibits release factor-dependent termination. Nat. Commun., 2022, 13(1): 2413.
- 22.Li D.; McDonald C.M.; Elfring G.L.; et al. Assessment of Treatment Effect With Multiple Outcomes in 2 Clinical Trials of Patients With Duchenne Muscular Dystrophy. JAMA Netw. Open, 2020, 3(2): e1921306.
- 23.McDonald C.M.; Muntoni F.; Penematsa V.; et al. Ataluren delays loss of ambulation and respiratory decline in nonsense mutation Duchenne muscular dystrophy patients. J. Comp. Eff. Res., 2022, 11(3): 139-55.
- 24.McDonald C.M.; Campbell C.; Torricelli R.E.; et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet, 2017, 390(10101): 1489-98.
- 25.Berger J.; Li M.; Berger S.; Effect of Ataluren on dystrophin mutations. J. Cell Mol Med., 2020, 24(12): 6680-9.
- 26.Wang B.; Li J.; Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. USA, 2000, 97(25): 13714-9.
- 27.Harper S.Q.; Hauser M.A.; DelloRusso C.; et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat. Med., 2002, 8(3): 253-61.
- 28.Duan D. Systemic AAV Micro-dystrophin Gene Therapy for Duchenne Muscular Dystrophy. Mol. Ther., 2018, 26(10): 2337-56.
- 29.Mendell J.R.; Sahenk Z;. Lehman K.; et al. Assessment of Systemic Delivery of rAAVrh74.MHCK7.micro-dystrophin in Children With Duchenne Muscular Dystrophy: A Nonrandomized Controlled Trial. JAMA Neurol. 2020, 77(9): 1122-31.
- 30.Sarepta Therapeutics I. Sarepta Therapeutics’ Investigational Gene Therapy SRP-9001 for Duchenne Muscular Dystrophy Demonstrates Significant Functional Improvements Across Multiple Studies. (accessed Oct 1,2022). https://www.globenewswire.com/news-release/2022/07/06/2474964/36419/en/Sarepta-Therapeutics-Investigational-Gene-Therapy-SRP-9001-for-Duchenne-Muscular-Dystrophy-Demonstrates-Significant-Functional-Improvements-Across-Multiple-Studies.html.
- 31.Pfizer I. Pfizer’s statement to the community. (accessed Oct 1,2022). https://www.parentprojectmd.org/wp-content/uploads/2021/12/DMD-Study-1001-Update_Letter-to-the-Community.pdf.
- 32.Pfizer I. Pfizer’s New Phase 1b Results of Gene Therapy in Ambulatory Boys with Duchenne Muscular Dystrophy (DMD) Support Advancement into Pivotal Phase 3 Study. (accessed Oct 1,2022). https://investors.pfizer.com/Investors/News/news-details/2020/Pfizers-New-Phase-1b-Results-of-Gene-Therapy-in-Ambulatory-Boys-with-Duchenne-Muscular-Dystrophy-DMD-Support-Advancement-into-Pivotal-Phase-3-Study-05-15-2020/default.aspx.
- 33.Pfizer I. A message from Pfizer on our DMD clinical program. (accessed Oct 1,2022). http://join.parentprojectmd.org/site/DocServer/A_Message_from_Pfizer_on_our_DMD_Clinical_Program_-_Sept.pdf.
- 34.Pagliarulo N. Pfizer aims to restart late-stage trial of Duchenne gene therapy following safety setback. (accessed Oct 1,2022). https://www.biopharmadive.com/news/pfizer-duchenne-phase-3-study-reopen/618642/.
- 35.Pfizer I. Pfizer announces clinical hold lifted for its mini-dystrophin gene therapy program. (accessed Oct 1,2022). https://www.parentprojectmd.org/wp-content/uploads/2022/04/Pfizer-Letter-to-the-Duchenne-community_April-28th-2022.pdf.
- 36.Markati T.; Oskoui M.; Farrar M.A.; et al. Emerging therapies for Duchenne muscular dystrophy. Lancet Neurol. 2022, 21(9): 814-29.
- 37.Le Guiner C.; Servais L.; Montus M.; et al. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat. Commun., 2017, 8: 16105.
- 38.Majowicz A.; Salas D.; Zabaleta N.; et al. Successful Repeated Hepatic Gene Delivery in Mice and Non-human Primates Achieved by Sequential Administration of AAV5 and AAV1. Mol. Ther., 2017, 25(8): 1831-42.
- 39.Leborgne C.; Barbon E.; Alexander J.M.; et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med., 2020, 26(7): 1096-101.
- 40.Olson E.N. Toward the correction of muscular dystrophy by gene editing. Proc. Natl. Acad. Sci. USA, 2021, 118(22).
- 41.Choi E.; Koo T. CRISPR technologies for the treatment of Duchenne muscular dystrophy. Mol. Ther., 2021, 29(11): 3179-91.
- 42.Johnson V. CRISPR Therapeutic Ready to Dose in Duchenne Muscular Dystrophy. (accessed Oct 1,2022). https://www.cgtlive.com/view/crispr-therapeutic-ready-to-dose-in-dmd.
- 43.Kenjo E.; Hozumi H.; Makita Y.; et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat. Commun., 2021, 12(1): 7101.
- 44.Newby G.A.; Liu D.R. In vivo somatic cell base editing and prime editing. Mol. Ther., 2021, 29(11): 3107-24.
- 45.Friedmann T.; Roblin R. Gene therapy for human genetic disease? Science, 1972, 175(4025): 949-55.
How to Cite
Hu, G.; Chen, C. Promising Treatments for Duchenne Muscular Dystrophy: Restoring Dystrophin Protein Expression Using Nucleic Acid Therapeutics. International Journal of Drug Discovery and Pharmacology 2023, 2 (1), 4–10. https://doi.org/10.53941/ijddp.0201002.
RIS
BibTex
Copyright & License

Guo Hu, Chen Chen
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


