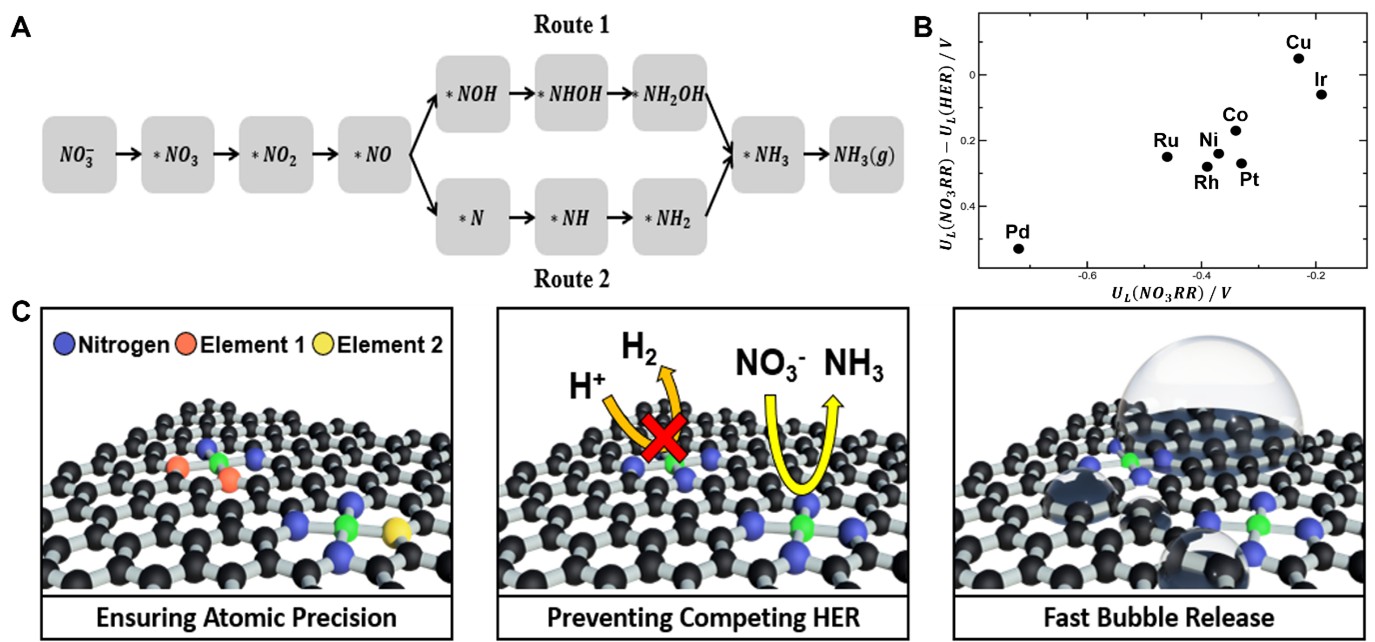
Ammonia is an essential industrial feedstock, but conventional synthesis processes, Haber-Bosch process, emit significant greenhouse gases. Electrochemical ammonia synthesis offers a promising alternative, enabling ammonia production without harmful pollutants. Recently, electrochemical nitrate reduction has attracted considerable attention for ammonia synthesis due to its high selectivity. Achieving efficient ammonia synthesis requires active and selective catalysts, and single-atom catalysts have shown great promise owing to their tunable chemical and geometric active sites. However, challenges remain, including the need to enhance active surface area and suppress competing hydrogen evolution reactions. This minireview summarizes recent advances in the design strategies of Cu-based single-atom catalysts, highlighting their advantages and performance in electrochemical nitrate reduction. Special emphasis is placed on the relationship between local composition/atomic structure and electrochemical nitrogen reduction performance by studying their local atomic coordination such as Cu-N4, Cu-NxO, CuM-Nx, and Cu embedded metal oxides. The review concludes by emphasizing the importance of further research to optimize Cu-based single-atom catalysts for efficient and sustainable ammonia production.
- Open Access
- Review
Cu-Based Atomic Catalysts for the Electrochemical Hydrogenation of Nitrate to Ammonia
- Hyeong Bin Park,
- Haneul Jin *
Author Information
Received: 22 Apr 2025 | Revised: 13 Jul 2025 | Accepted: 26 Aug 2025 | Published: 20 Nov 2025
Abstract
Graphical Abstract
References
- 1.Jin, H.; Kim, S.S.; Venkateshalu, S.; et al. Electrochemical Nitrogen Fixation for Green Ammonia: Recent Progress and Challenges. Adv. Sci. 2023, 10, 2300951. https://doi.org/10.1002/advs.202300951.
- 2.Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. https://doi.org/10.1021/acs.energyfuels.0c03685.
- 3.Hasan, M.H.; Mahlia TM, I.; Mofijur, M.; et al. A comprehensive review on the recent development of ammonia as a renewable energy carrier. Energies 2021, 14, 3732. https://doi.org/10.3390/en14133732.
- 4.Chyong, C.K.; Italiani, E.; Kazantzis, N. Energy and climate policy implications on the deployment of low-carbon ammonia technologies. Nat. Commun. 2025, 16, 776. https://doi.org/10.1038/s41467-025-56006-6.
- 5.El-Shafie, M.; Kambara, S. Recent advances in ammonia synthesis technologies: Toward future zero carbon emissions. Int. J. Hydrogen Energy 2023, 48, 11237–11273. https://doi.org/10.1016/j.ijhydene.2022.09.061.
- 6.Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021, 5, 2814–2839. https://doi.org/10.1039/D1SE00345C.
- 7.Wang, B.; Li, T.; Gong, F.; et al. Ammonia as a green energy carrier: Electrochemical synthesis and direct ammonia fuel cell—A comprehensive review. Fuel Process. Technol. 2022, 235, 107380. https://doi.org/10.1016/j.fuproc.2022.107380.
- 8.Kojima, Y.; Yamaguchi, M. Ammonia as a hydrogen energy carrier. Int. J. Hydrog. Energy 2022, 47, 22832–22839. https://doi.org/10.1016/j.ijhydene.2022.05.096.
- 9.Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. https://doi.org/10.1002/aesr.202000043.
- 10.Erfani, N.; Baharudin, L.; Watson, M. Recent advances and intensifications in Haber-Bosch ammonia synthesis process. Chem. Eng. Process. Process Intensif. 2024, 204, 109962. https://doi.org/10.1016/j.cep.2024.109962.
- 11.Appl, M. The Haber-Bosch heritage: The ammonia production technology. In Proceedings of the 50th Anniversary of the IFA Technical Conference, Sevilla, Spain, 25–26 September 1997.
- 12.Kim, H.S.; Jin, H.; Kim, S.H.; et al. Sacrificial Dopant to Enhance the Activity and Durability of Electrochemical N2 Reduction Catalysis. ACS Catal. 2022, 12, 5684–5697. https://doi.org/10.1021/acscatal.2c00089.
- 13.Jin, H.; Kim, H.S.; Lee, C.H.; et al. Directing the Surface Atomic Geometry on Copper Sulfide for Enhanced Electrochemical Nitrogen Reduction. ACS Catal. 2022, 12, 13638–13648. https://doi.org/10.1021/acscatal.2c03680.
- 14.Mingolla, S.; Rosa, L. Low-carbon ammonia production is essential for resilient and sustainable agriculture. Nat. Food 2025, 6, 610–621. https://doi.org/10.1038/s43016-025-01125-y.
- 15.Hollevoet, L.; De Ras, M.; Roeffaers, M.; et al. A. Energy-Efficient Ammonia Production from Air and Water Using Electrocatalysts with Limited Faradaic Efficiency. ACS Energy Lett. 2020, 5, 1124–1127. https://doi.org/10.1021/acsenergylett.0c00455.
- 16.Tang, C.; Qiao, S.-Z. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem. Soc. Rev. 2019, 48, 3166–3180. https://doi.org/10.1039/C9CS00280D.
- 17.Zhang, X.; Wang, Y.; Liu, C.; et al. Recent advances in non-noble metal electrocatalysts for nitrate reduction. Chem. Eng. J. 2021, 403, 126269. https://doi.org/10.1016/j.cej.2020.126269.
- 18.Jia, S.; Sun, X.; Han, B. Electrocatalytic systems for NOx upgrading. Chem. Commun. 2025, 61, 1262–1274. https://doi.org/10.1039/D4CC05762G.
- 19.Wang, D.; Lu, X.F.; Luan, D.; et al. Selective Electrocatalytic Conversion of Nitric Oxide to High Value-Added Chemicals. Adv. Mater. 2024, 36, 2312645. https://doi.org/10.1002/adma.202312645.
- 20.Chen, G.F.; Yuan, Y.; Jiang, H.; et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. https://doi.org/10.1038/s41560-020-0654-1.
- 21.Deng, X.; Yang, Y.; Wang, L.; et al. Metallic Co Nanoarray Catalyzes Selective NH3 Production from Electrochemical Nitrate Reduction at Current Densities Exceeding 2 A cm−2. Adv. Sci. 2021, 8, 2004523. https://doi.org/10.1002/advs.202004523.
- 22.McEnaney, J.M.; Blair, S.J.; Nielander, A.C.; et al. Electrolyte Engineering for Efficient Electrochemical Nitrate Reduction to Ammonia on a Titanium Electrode. ACS Sustain. Chem. Eng. 2020, 8, 2672–2681. https://doi.org/10.1021/acssuschemeng.9b05983.
- 23.Bai, L.; Franco, F.; Timoshenko, J.; et al. Electrocatalytic Nitrate and Nitrite Reduction toward Ammonia Using Cu2O Nanocubes: Active Species and Reaction Mechanisms. J. Am. Chem. Soc. 2024, 146, 9665–9678. https://doi.org/10.1021/jacs.3c13288.
- 24.Karamad, M.; Goncalves, T.J.; Jimenez-Villegas, S.; et al. Why copper catalyzes electrochemical reduction of nitrate to ammonia. Faraday Discuss. 2023, 243, 502–519. https://doi.org/10.1039/D2FD00145D.
- 25.Choi, J.; Suryanto BH, R.; Wang, D.; et al. Identification and elimination of false positives in electrochemical nitrogen reduction studies. Nat. Commun. 2020, 11, 5546. https://doi.org/10.1038/s41467-020-19130-z.
- 26.Liao, G.; Smith, R.L., Jr.; Guo, H.; et al. Review of carbon-based catalysts for electrochemical nitrate reduction and green ammonia synthesis. Green Chem. 2024, 26, 11797–11831. https://doi.org/10.1039/D4GC04640D.
- 27.Liao, W.; Wang, J.; Ni, G.; et al. Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm−2. Nat. Commun. 2024, 15, 1264. https://doi.org/10.1038/s41467-024-45534-2.
- 28.Hu, T.; Wang, C.; Wang, M.; et al. Theoretical Insights into Superior Nitrate Reduction to Ammonia Performance of Copper Catalysts. ACS Catal. 2021, 11, 14417–14427. https://doi.org/10.1021/acscatal.1c03666.
- 29.Fang, L.; Wang, S.; Song, C.; et al. Boosting nitrate electroreduction to ammonia via in situ generated stacking faults in oxide-derived copper. Chem. Eng. J. 2022, 446, 137341. https://doi.org/10.1016/j.cej.2022.137341.
- 30.Chen, H.; Zhang, C.; Sheng, L.; et al. Copper single-atom catalyst as a high-performance electrocatalyst for nitrate-ammonium conversion. J. Hazard. Mater. 2022, 434, 128892. https://doi.org/10.1016/j.jhazmat.2022.128892.
- 31.Murphy, E.; Liu, Y.; Matanovic, I.; et al. Elucidating electrochemical nitrate and nitrite reduction over atomically-dispersed transition metal sites. Nat. Commun. 2023, 14, 4554. https://doi.org/10.1038/s41467-023-40174-4.
- 32.Long, X.; Zhong, T.; Huang, F.; et al. Exploring microenvironmental configuration effects of Cu-based catalysts on nitrate electrocatalytic reduction selectivity. Appl. Catal. B Environ. Energy 2025, 365, 124944. https://doi.org/10.1016/j.apcatb.2024.
- 33.Wang, Y.; Dutta, A.; Iarchuk, A.; et al. Boosting Nitrate to Ammonia Electroconversion through Hydrogen Gas Evolution over Cu-foam@mesh Catalysts. ACS Catal. 2023, 13, 8169–8182. https://doi.org/10.1021/acscatal.3c00716.
- 34.Fan, K.; Xie, W.; Li, J.; et al. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 2022, 13, 7958. https://doi.org/10.1038/s41467-022-35664-w.
- 35.Liu, Y.; Qiu, W.; Wang, P.; et al. Pyridine-N-rich Cu single-atom catalyst boosts nitrate electroreduction to ammonia. Appl. Catal. B Environ. 2024, 340, 123228. https://doi.org/10.1016/j.apcatb.2023.123228.
- 36.Zhu, T.; Chen, Q.; Liao, P.; et al. Single-Atom Cu Catalysts for Enhanced Electrocatalytic Nitrate Reduction with Significant Alleviation of Nitrite Production. Small 2020, 16, 2004526. https://doi.org/10.1002/smll.202004526.
- 37.Yang, J.; Qi, H.; Li, A.; et al. Potential-Driven Restructuring of Cu Single Atoms to Nanoparticles for Boosting the Electrochemical Reduction of Nitrate to Ammonia. J. Am. Chem. Soc. 2022, 144, 12062–12071. https://doi.org/10.1021/jacs.2c02262.
- 38.Gu, Z.; Zhang, Y.; Fu, Y.; et al. Coordination Desymmetrization of Copper Single-Atom Catalyst for Efficient Nitrate Reduction. Angew. Chem. 2024, 136, e202409125. https://doi.org/10.1002/ange.202409125.
- 39.Cheng, X.F.; He, J.H.; Ji, H.Q.; et al. Coordination Symmetry Breaking of Single-Atom Catalysts for Robust and Efficient Nitrate Electroreduction to Ammonia. Adv. Mater. 2022, 34, 2205767. https://doi.org/10.1002/adma.202205767.
- 40.Zhang, S.; Wu, J.; Zheng, M.; et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 14, 3634. https://doi.org/10.1038/s41467-023-39366-9.
- 41.Wang, Y.; Yin, H.; Dong, F.; et al. N‐coordinated Cu–Ni dual‐single‐atom catalyst for highly selective electrocatalytic reduction of nitrate to ammonia. Small 2023, 19, 2207695. https://doi.org/10.1002/smll.202207695.
- 42.Shen, F.; He, S.; Tang, X.; et al. Breaking Linear Scaling Relation Limitations on a Dual-Driven Single-Atom Copper-Tungsten Oxide Catalyst for Ammonia Synthesis. Angew. Chem. Int. Ed. 2025, 64, e202423154. https://doi.org/10.1002/anie.202423154.
- 43.Liu, Y.; Wei, J.; Yang, Z.; et al. Efficient tandem electroreduction of nitrate into ammonia through coupling Cu single atoms with adjacent Co3O4. Nat. Commun. 2024, 15, 3619. https://doi.org/10.1038/s41467-024-48035-4.
- 44.Zhao, X.; Hu, G.; Chen, G.F.; et al. Comprehensive Understanding of the Thriving Ambient Electrochemical Nitrogen Reduction Reaction. Adv. Mater. 2021, 33, 2007650. https://doi.org/10.1002/adma.202007650.
- 45.Kundu, J.; Bhoyar, T.; Park, S.; et al. Recent advances in single- and dual-atom catalysts for efficient nitrogen electro-reduction and their perspectives. Adv. Powder Mater. 2025, 4, 100279. https://doi.org/10.1016/j.apmate.2025.100279.
- 46.Xue, Y.; Yu, Q.; Ma, Q.; et al. Electrocatalytic Hydrogenation Boosts Reduction of Nitrate to Ammonia over Single-Atom Cu with Cu(I)-N3C1 Sites. Environ. Sci. Technol. 2022, 56, 14797–14807. https://doi.org/10.1021/acs.est.2c04456.
- 47.Cheng, J.; Sun, W.; Dai, G.; et al. Electroreduction of nitrate to ammonia on atomically-dispersed Cu-N4 active sites with high efficiency and stability. Fuel 2023, 332, 126106. https://doi.org/10.1016/j.fuel.2022.126106.
- 48.Huang, T.; Liang, T.; You, J.; et al. Coordination environment-tailored electronic structure of single atomic copper sites for efficient electrochemical nitrate reduction toward ammonia. Energy Environ. Sci. 2024, 17, 8360–8367. https://doi.org/10.1039/D4EE02746A.
- 49.Li, Y.; Lu, Z.; Zheng, L.; et al. The synergistic catalysis effect on electrochemical nitrate reduction at the dual-function active sites of the heterostructure. Energy Environ. Sci. 2024, 17, 4582–4593. https://doi.org/10.1039/D4EE00784K.
- 50.Yin, H.; Peng, Y.; Li, J. Electrocatalytic Reduction of Nitrate to Ammonia via a Au/Cu Single Atom Alloy Catalyst. Environ. Sci. Technol. 2023, 57, 3134–3144. https://doi.org/10.1021/acs.est.2c07968.
- 51.Du, C.; Lu, S.; Wang, J.; et al. Selectively Reducing Nitrate into NH3 in Neutral Media by PdCu Single-Atom Alloy Electrocatalysis. ACS Catal. 2023, 13, 10560–10569. https://doi.org/10.1021/acscatal.3c01088.
- 52.Cai, J.; Wei, Y.; Cao, A.; et al. Electrocatalytic nitrate-to-ammonia conversion with ~100% Faradaic efficiency via single-atom alloying. Appl. Catal. B Environ. 2022, 316, 121683. https://doi.org/10.1016/j.apcatb.2022.121683.
- 53.Yu, J.; Gao, R.T.; Guo, X.; et al. Electrochemical Nitrate Reduction to Ammonia on AuCu Single-Atom Alloy Aerogels under Wide Potential Window. Angew. Chem. Int. Ed. 2025, 64, e202415975. https://doi.org/10.1002/anie.202415975.
- 54.Suh, J.; Choi, H.; Kong, Y.; et al. Tandem Electroreduction of Nitrate to Ammonia Using a Cobalt–Copper Mixed Single-Atom/Cluster Catalyst with Synergistic Effects. Adv. Sci. 2024, 11, 2407250. https://doi.org/10.1002/advs.202407250.
- 55.Jiang, G.; Liu, Z.; He, S.; et al. Single-Atom Copper-Bearing Cerium Oxide Electrocatalysts Embedded in an Integrated System Enable Sustainable Nitrogen Recycling from Natural Water Bodies. ACS EST Eng. 2024, 4, 2912–2922. https://doi.org/10.1021/acsestengg.4c00299.
- 56.Liu, Z.; Shen, F.; Shi, L.; et al. Electronic Structure Optimization and Proton-Transfer Enhancement on Titanium Oxide-Supported Copper Nanoparticles for Enhanced Nitrogen Recycling from Nitrate-Contaminated Water. Environ. Sci. Technol. 2023, 57, 10117–10126. https://doi.org/10.1021/acs.est.3c03431.
- 57.Fang, L.; Wang, S.; Lu, S.; et al. Efficient electroreduction of nitrate via enriched active phases on copper-cobalt oxides. Chin. Chem. Lett. 2024, 35, 108864. https://doi.org/10.1016/j.cclet.2023.108864.
- 58.Wang, J.; Wang, Y.; Cai, C.; et al. Cu-Doped Iron Oxide for the Efficient Electrocatalytic Nitrate Reduction Reaction. Nano Lett. 2023, 23, 1897–1903. https://doi.org/10.1021/acs.nanolett.2c04949.
- 59.Zhou, B.; Tong, Y.; Yao, Y.; et al. Reversed I1Cu4 single-atom sites for superior neutral ammonia electrosynthesis with nitrate. Proc. Natl. Acad. Sci. USA 2024, 121, e2405236121. https://doi.org/10.1073/pnas.2405236121.
- 60.Shi, L.; Li, Y.; He, S.; et al. Efficient electrocatalytic nitrate reduction on molecular catalyst with electron-deficient single-atom Cuδ+ sites. Chem. Eng. J. 2024, 495, 153427. https://doi.org/10.1016/j.cej.2024.153427.
- 61.Wang, Y.; Xia, S.; Zhang, J.; et al. Halogen-induced planar defects in Cu catalysts for ammonia electrosynthesis at an ampere-level current density. Mater. Chem. Front. 2023, 7, 3093–3101. https://doi.org/10.1039/D3QM00114H.
How to Cite
Park, H. B.; Jin, H. Cu-Based Atomic Catalysts for the Electrochemical Hydrogenation of Nitrate to Ammonia. Materials and Sustainability 2025, 1 (4), 14. https://doi.org/10.53941/matsus.2025.100014.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


