This review provides a comprehensive overview of the structural transformation from amorphous carbon—particularly in graphitizable soft carbons—to ordered graphitic crystalline planes, emphasizing the underlying mechanisms, advanced characterization techniques, and diverse applications. Carbon materials exhibit a broad spectrum of structures, from disordered amorphous forms to highly crystalline allotropes like graphite and graphene, with controlled transitions enabling tailored properties for energy storage, electronics, sensors, and composites. Key graphitization methods, including thermal annealing, catalytic processes, pressure-assisted techniques, and irradiation, are discussed in relation to processing conditions and resulting nanostructures. Thermodynamic and kinetic considerations, structural reorganization pathways, and the influence of heteroatoms and impurities are explored in depth. State-of-the-art characterization tools such as XRD, Raman spectroscopy, and TEM offer insights into atomic-scale studies. The review also addresses current challenges, emerging trends like sustainable and energy-efficient approaches, and future prospects for innovative carbon-based technologies.
- Open Access
- Review
An Overview of the Transition from Amorphous Carbon to an Ordered Graphitic Crystalline Plane for Applications
- Adriana Montiel-García 1,
- Manoj Kumar Srinivasan 2,
- Ignacio Avila Aguilar 1,
- Wilgince Apollon 1,
- Arun Thirumurugan 3,
- Sathish-Kumar Kamaraj 1, *
Author Information
Received: 07 Aug 2025 | Revised: 14 Sep 2025 | Accepted: 17 Sep 2025 | Published: 30 Sep 2025
Abstract
Graphical Abstract
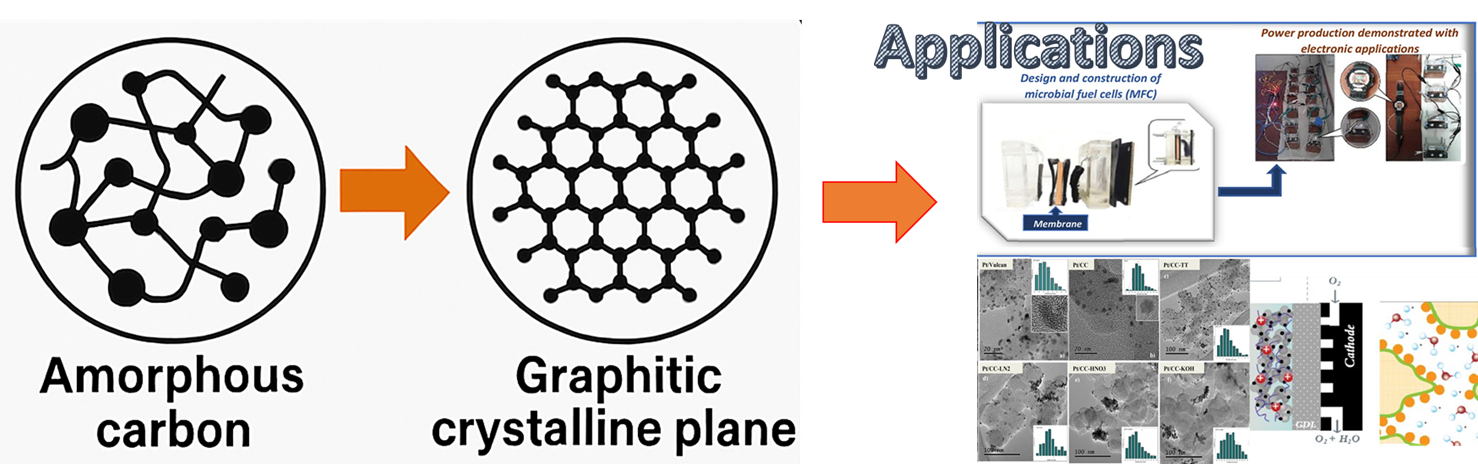
Keywords
amorphous carbon | energy barriers | hybridization | thermal annealing
References
- 1.Yadav, C.S.; Azad, I.; Khan, A.R.; et al. Carbon allotropes: Past to present aspects. In Biosensors Based on Graphene, Graphene Oxide and Graphynes for Early Detection of Cancer; CRC Press: Boca Raton, FL, USA, 2025; pp. 1–23.
- 2.Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859.
- 3.Sabina-Delgado, A.; Kamaraj, S.K.; Hernández-Montoya, V.; et al. Novel carbon-ceramic composite membranes with high cation exchange properties for use in microbial fuel cell and electricity generation. Int J Hydrogen Energy 2023, 48, 25512–25526.
- 4.Kang, J.; Yang, X.; Hu, Q.; et al. Recent progress of amorphous nanomaterials. Chem. Rev. 2023, 123, 8859–8941.
- 5.Duan, X.; Tian, W.; Zhang, H.; et al. sp2/sp3 framework from diamond nanocrystals: A key bridge of carbonaceous structure to carbocatalysis. ACS Catal. 2019, 9, 7494–7519.
- 6.Ike, S.; Vander Wal, R. Effect of carbonization methods on graphitization of soft and hard carbons. Carbon Trends 2024, 16, 100382. https://doi.org/10.1016/j.cartre.2024.100382.
- 7.Pozio, A.; Di Carli, M.; Aurora, A.; et al. Hard Carbons for Use as Electrodes in Li-S and Li-ion Batteries. Nanomaterials 2022, 12, 1349. https://doi.org/10.3390/nano12081349.
- 8.Ren, X.; Hussain, M.I.; Chang, Y.; et al. State-of-the-Art review on amorphous carbon nanotubes: Synthesis, structure, and application. Int. J. Mol. Sci. 2023, 24, 17239.
- 9.Tejasvi, R. Properties of Carbon-Based Nanomaterials and Techniques for Characterization. In Carbon-Based Nanomaterials for Green Applications; Wiley: Hoboken, NJ, USA, 2024; pp. 21–55.
- 10.Ugwumadu, C.; Olson, R. III; Smith, N.L.; et al. Computer simulation of carbonization and graphitization of coal. Nanotechnology 2023, 35, 095703.
- 11.Ouzilleau, P.; Gheribi, A.; Chartrand, P. The graphitization temperature threshold analyzed through a second-order structural transformation. Carbon 2016, 109, 896–908.
- 12.Goswami, A.D.; Trivedi, D.H.; Jadhav, N.L.; et al. Sustainable and green synthesis of carbon nanomaterials: A review. J. Environ. Chem. Eng. 2021, 9, 106118.
- 13.Tu, J.; Wang, X.; Jiang, L.; et al. Efficient graphitization conversion strategies of low-value carbonaceous resources into advanced graphitic carbons. Chem. Eng. J. 2025, 505, 159472.
- 14.Aswathappa, S.; Dhas, S.S.; Kumar, R.S. Acoustic shock wave-induced sp2-to-sp3-type phase transition-part II: Evidence of the presence of diamond from valance band spectra and electronic diffraction pattern. Diam. Relat. Mater. 2024, 150, 111680.
- 15.Chen, C.; Xie, L. Graphene and Graphdiyne. In Carbon Catalysis; CRC Press: Boca Raton, FL, USA, 2024; pp. 149–214.
- 16.Sheka, E.F.; Golubev, Y.A.; Popova, N.A. Amorphous state of sp2 solid carbon. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 107–113.
- 17.Basit, M.A.; Zafar, R.; Haider, N. Properties of Diamond-like Carbon Coatings. Appl. Diam.-Like Carbon Coat. 2025, 4, 71–106.
- 18.Tripathi, N.; Sharma, P.; Pavelyev, V.; et al. A detailed study on carbon nanotubes: Properties, synthesis, and characterization. In Chemically Modified Carbon Nanotubes for Commercial Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 1–49.
- 19.Reyes-Rodríguez, J.L.; Sathish-Kumar, K.; Solorza-Feria, O. Synthesis and functionalization of green carbon as a Pt catalyst support for the oxygen reduction reaction. Int J Hydrogen Energy 2015, 40, 17253–17263.
- 20.Diaz, J.; Monteiro, O.R.; Hussain, Z.; Structure of amorphous carbon from near-edge and extended X-ray absorption spectroscopy. Phys. Rev. B. 2007, 76, 94201. https://doi.org/10.1103/PhysRevB.76.094201.
- 21.Phua, E.J.; Kai, T.Y.; Woon, L.Y.; et al. Ultra-Thin ta-C Hermetic Seals for Electronics Packaging. In Proceedings of the 2024 IEEE 26th Electronics Packaging Technology Conference (EPTC), Singapore, 3–6 December 2024; pp. 828–835.
- 22.Shunin, Y.; Bellucci, S.; Gruodis, A.; et al. General Approach to the Description of Fundamental Properties of Disordered Nanosized Media. In Nonregular Nanosystems: Theory and Applications; Springer International Publishing: Cham, Switzerland, 2017; pp. 7–31.
- 23.Odusanya, A.; Rahaman, I.; Sarkar, P.K.; et al. Laser-Assisted Growth of Carbon-Based Materials by Chemical Vapor Deposition. C 2022, 8, 24.
- 24.Li, J.; Yin, D.; Qin, Y. Carbon materials: Structures, properties, synthesis and applications. Manuf. Rev. 2023, 10, 13.
- 25.Obadero, A.S. Intercalation in Graphite Materials. Ph.D. Thesis, Université Grenoble Alpes, Grenoble, France, 2020.
- 26.Yang, P.J.; Li, T.H.; Li, H.; et al. Progress in the graphitization and applications of modified resin carbons. New Carbon Mater. 2023, 38, 96–108.
- 27.Cao, D.; Wang, L.; Ding, Z.; et al. Characterization of the heterogeneous evolution of the nanostructure of coal-based graphite. J. Nanosci. Nanotechnol. 2021, 21, 670–681.
- 28.Hunter, R.D.; Ramírez-Rico, J.; Schnepp, Z. Iron-catalyzed graphitization for the synthesis of nanostructured graphitic carbons. J. Mater. Chem. A 2022, 10, 4489–4516.
- 29.Sun, J.; Dang, Y.; Sun, X.; et al. Can carbon be used as an anode for water splitting? ChemSusChem 2025, 18, e202401340.
- 30.Ghosh, S.; Zaid, M.; Dutta, J.; et al. Soft carbon in non-aqueous rechargeable batteries: A review of its synthesis, carbonization mechanism, characterization, and multifarious applications. Energy Adv. 2024, 3, 1167–1195. https://doi.org/10.1039/D4YA00174E.
- 31.Presser, V.; Heon, M.; Gogotsi, Y. Carbide-derived carbons–from porous networks to nanotubes and graphene. Adv. Funct. Mater. 2011, 21, 810–833.
- 32.Bhattacharyya, S. Carbon Superstructures: From Quantum Transport to Quantum Computation; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–326.
- 33.Yuan, G.; Li, B.; Li, X.; et al. Effect of Liquid Crystalline Texture of Mesophase Pitches on the Structure and Property of Large-Diameter Carbon Fibers. ACS Omega 2019, 4, 1095–1102. https://doi.org/10.1021/acsomega.8b03189.
- 34.Li, H.; Li, X.; Wei, J.; et al. Crystalline transformation from ta-C to graphene induced by a catalytic Ni layer during annealing. Diam. Relat. Mater. 2020, 101, 107556.
- 35.Loh, G.C.; Baillargeat, D. Graphitization of amorphous carbon and its transformation pathways. J. Appl. Phys. 2013, 114, 033534.
- 36.Zhao, Y.; Zhao, C.; Wang, X.; et al. Stability of sp Hybridized Amorphous Carbon and its Transformation to Nanodiamond. Small Methods 2025, 9, 2500294.
- 37.Konicek, A.R.; Grierson, D.S.; Gilbert, P.U.P.A.; et al. Origin of Ultralow Friction and Wear in Ultrananocrystalline Diamond. Phys. Rev. Lett. 2008, 100, 235502.
- 38.Thapa, R.; Ugwumadu, C.; Nepal, K.; et al. Ab Initio Simulation of Amorphous Graphite. Phys. Rev. Lett. 2022, 128, 236402.
- 39.Gomez-Martin, A.; Schnepp, Z.; Ramirez-Rico, J. Structural Evolution in Iron-Catalyzed Graphitization of Hard Carbons. Chem. Mater. 2021, 33, 3087–3097.
- 40.Kupka, K.; Leino, A.; Ren, W.; et al. Graphitization of Amorphous Carbon by Swift Heavy Ion Impacts: Molecular Dynamics Simulation. Diam. Relat. Mater. 2018, 83, 134–140.
- 41.Li, K.; Zhang, H.; Li, G.; et al. ReaxFF Molecular Dynamics Simulation for the Graphitization of Amorphous Carbon: A Parametric Study. J. Chem. Theory Comput. 2018, 14, 2322–2331.
- 42.Liu, Y.; Gao, T.; Xiao, Q.; et al. Generalized modeling of carbon film deposition growth via hybrid MD/MC simulations with machine-learning potentials. NPJ Comput. Mater. 2025, 11, 285.
- 43.Amini, S.; Abbaschian, R. Nucleation and growth kinetics of graphene layers from a molten phase. Carbon 2013, 51, 110–123. https://doi.org/10.1016/j.carbon.2012.08.019.
- 44.Boubiche, N.; El Hamouchi, J.; Hulik, J.; et al. Kinetics of Graphitization of thin diamond-like carbon (DLC) films catalyzed by transition metal. Diam. Relat. Mater. 2019, 91, 190–198.
- 45.Rigollet, S.; Weiss-Hortala, E.; Flamant, G.; et al. Biocarbon graphenization processes and energy assessment. A Rev. Chem. Eng. J. 2024, 496, 153795. https://doi.org/10.1016/j.cej.2024.153795.
- 46.Thambiliyagodage, C.J.; Ulrich, S.; Araujo, P.T.; et al. Catalytic graphitization in nanocast carbon monoliths by iron, cobalt and nickel nanoparticles. Carbon 2018, 134, 452–463.
- 47.Abdullah, N.R.; Rashid, H.O.; Tang, C.S.; et al. Controlling physical properties of bilayer graphene by stacking orientation caused by interaction between B and N dopant atoms. Mater. Sci. Eng. B 2022, 276, 115554.
- 48.Liu, C.; Fang, W.; Cheng, Q.; et al. Revolutionizing elastomer technology: Advances in reversible crosslinking, reprocessing, and self-healing applications. Polym. Rev. 2025, 65, 483–526.
- 49.Wang, M.X. Nitrogen and oxygen bridged calixaromatics: Synthesis, structure, functionalization, and molecular recognition. Acc. Chem. Res. 2012, 45, 182–195.
- 50.McLean, B.; Webber, G.; Page, A. Boron Nitride Nucleation Mechanism during Chemical Vapor Deposition. J. Phys. Chem. C 2018, 122, 24341–24349. https://doi.org/10.1021/acs.jpcc.8b05785.
- 51.Fan, X.; Dong, X.; Wei, W.H.; et al. Monitoring single-heteroatom loss during deoxygenation and denitrogenation of soluble organic matter in coal using mass spectrometric methods. Fuel 2021, 292, 120294.
- 52.Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767.
- 53.McKee, D.W. Oxidation protection of carbon materials. In Chemistry & Physics of Carbon; Boca Raton, FL, USA, 2021; pp. 173–232.
- 54.Li, S.; Liu, J.; Chen, Y.; et al. Graphitization Induction Effect of Hard Carbon for Sodium-Ion Storage. Adv. Funct. Mater. 2025, 35, 2424629.
- 55.Devi, M.; Wang, H.; Moon, S.; et al. Laser-Carbonization–A powerful tool for micro-fabrication of patterned electronic carbons. Adv. Mater. 2023, 35, 2211054.
- 56.Inagaki, M.; Kaburagi, Y.; Hishiyama, Y. Thermal management material: Graphite. Adv. Eng. Mater. 2014, 16, 494–506.
- 57.Ren, K.; Liu, Z.; Wei, T.; et al. Recent developments of transition metal compounds-carbon hybrid electrodes for high energy/power supercapacitors. Nano-Micro Lett. 2021, 13, 129.
- 58.Goronzy, D.P.; Ebrahimi, M.; Rosei, F.; et al. Supramolecular assemblies on surfaces: Nanopatterning, functionality, and reactivity. ACS Nano 2018, 12, 7445–7481.
- 59.Izatt, R.M.; Izatt, S.R.; Izatt, N.E.; et al. Industrial applications of molecular recognition technology to separations of platinum group metals and selective removal of metal impurities from process streams. Green Chem. 2015, 17, 2236–2245.
- 60.Xu, R.; Du, L.; Adekoya, D.; et al. Well-defined nanostructures for electrochemical energy conversion and storage. Adv. Energy Mater. 2021, 11, 2001537.
- 61.Li, X.Y.; Zhang, Z.H.; Cheng, X.W.; et al. The development and application of spark plasma sintering technique in advanced metal structure materials: A review. Powder Metall. Met. Ceram. 2021, 60, 410–438.
- 62.Zhu, W.; Zhu, L. Argon-assisted electrical explosion of graphite powder in a constraint tube: Experimental and MD insights into the exfoliation mechanism. Ceram. Int. 2025, 51, 21689–21701.
- 63.Taqy, S.; Haque, A. Radiation-induced synthesis of carbon nanostructures. In Handbook of Functionalized Carbon Nanostructures: From Synthesis Methods to Applications; Springer International Publishing: Cham, Switzerland, 2024; pp. 729–788.
- 64.Sathish-Kumar, K.; Vázquez-Huerta, G.; Rodríguez-Castellanos, A.; et al. Microwave Assisted Synthesis and Characterizations of Decorated Activated Carbon. Int. J. Electrochem. Sci. 2012, 7, 5484–5494.
- 65.Krasheninnikov, A.V.; Banhart, F.J. Engineering of nanostructured carbon materials with electron or ion beams. Nat. Mater. 2007, 6, 723–733.
- 66.Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and modification of carbon nanomaterials utilizing microwave heating. Adv. Mater. 2015, 27, 4113–4141.
- 67.Ramasundaram, S.; Jeevanandham, S.; Vijay, N.; et al. Unraveling the Dynamic Properties of New-Age Energy Materials Chemistry Using Advanced In Situ Transmission Electron Microscopy. Molecules 2024, 29, 4411.
- 68.Nakamura, Y.; Yoshino, T.; Satish-Kumar, M. Pressure dependence of graphitization: Implications for rapid recrystallization of carbonaceous material in a subduction zone. Contrib. Mineral. Petrol. 2020, 175, 32.
- 69.Mishra, S.; Datta, R. Embrittlement of Steel. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., et al., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 2761–2768.
- 70.Mostafavi, E.; Iravani, S.; Varma, R.S.; et al. Eco-friendly synthesis of carbon nanotubes and their cancer theranostic applications. Mater. Adv. 2022, 3, 4765–4782.
- 71.Choi, G.B.; Ahn, J.-R.; Kim, J.; et al. Unraveling the Catalytic Graphitization Mechanism of Ni–P Electroless Plated Cokes via In Situ Analytical Approaches. ACS Omega 2024, 9, 6741–6748.
- 72.Kuzovkov, V.; Kotomin, E.; Vila, R. Theoretical analysis of thermal annealing kinetics of radiation defects in silica. J. Nucl. Mater. 2023, 579, 154381. https://doi.org/10.1016/j.jnucmat.2023.154381.
- 73.Xu, X.; Cao, D.; Wei, Y.; et al. Impact of Graphitization Degree on the Electrochemical and Thermal Properties of Coal. ACS Omega 2024, 9, 2443–2456. https://doi.org/10.1021/acsomega.3c06871.
- 74.Yan, Q.; Xin, Y.; Zhang, X.; et al. Effect of graphitization temperature on microstructure, mechanical and ablative properties of C/C composites with pitch and pyrocarbon dual-matrix. Ceram. Int. 2022, 49, 2860–2870. https://doi.org/10.1016/j.ceramint.2022.09.269.
- 75.Chen, C.; Sun, K.; Wang, A.; et al. Catalytic graphitization of cellulose using nickel as catalyst. BioResources 2018, 13, 3165–3176.
- 76.Jeon, C.; Hwang, S.; Han, M.; et al. Unveiling the graphitization behaviors of highly stiff and thermally conductive graphitic carbon fibers. Carbon 2025, 245, 120791.
- 77.Shokrani Havigh, R.; Mahmoudi Chenari, H. A comprehensive study on the effect of carbonization temperature on the physical and chemical properties of carbon fibers. Sci. Rep. 2022, 12, 10704. https://doi.org/10.1038/s41598-022-15085-x.
- 78.Clarke, A.P. Catalytic Methane Chemistry in High-Temperature Molten Environments. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2021.
- 79.Pusarapu, V.; Narayana Sarma, R.; Ochonma, P.; et al. Sustainable co-production of porous graphitic carbon and synthesis gas from biomass resources. NPJ Mater. Sustain. 2024, 2, 16. https://doi.org/10.1038/s44296-024-00020-0.
- 80.Lower, L.; Dey, S.; Vook, T.; et al. Catalytic Graphitization of Biocarbon for Lithium-Ion Anodes: A Minireview. ChemSusChem 2023, 16, e202300729.
- 81.Liu, P.; Du, W.; Liu, X.; et al. Sustainable catalytic graphitization of biomass to graphitic porous carbon by constructing permeation network with organic ligands. Chin. J. Chem. Eng. 2023, 64, 259–270. https://doi.org/10.1016/j.cjche.2023.06.025.
- 82.Shi, M.; Song, C.; Tai, Z.; et al. Coal-derived synthetic graphite with high specific capacity and excellent cyclic stability as anode material for lithium-ion batteries. Fuel 2021, 292, 120250. https://doi.org/10.1016/j.fuel.2021.120250.
- 83.Tung, T.T.; Pereira, A.L.C.; Poloni, E.; et al. Irradiation methods for engineering of graphene related two-dimensional materials. Appl. Phys. Rev. 2023, 10, 031309. https://doi.org/10.1063/5.0148376.
- 84.Frankenstein, L.; Glomb, P.; Ramirez-Rico, J.; et al. Revealing the Impact of Different Iron-Based Precursors on the ‘Catalytic’ Graphitization for Synthesis of Anode Materials for Lithium Ion Batteries. ChemElectroChem 2023, 10, e202201073.
- 85.Shang, T.; Zhan, H.; Gong, Q.; et al. Insights into the thermal and electric field distribution and the structural optimization in the graphitization furnace. Energy 2024, 297, 131269.
- 86.Acedera, R. How Does Graphitization Affect the Structure and Properties of Carbon Fiber Papers? 2023. Available online: https://blog.caplinq.com/how-does-graphitization-affect-the-structure-and-properties-of-carbon-fiber-papers_4869/ (accessed on 14 September 2025).
- 87.Yoshihiro, N.; Takashi, Y.; Sathish, M. An experimental kinetic study on the structural evolution of natural carbonaceous material to graphite. Am. Mineral. 2017, 102, 135–148
- 88.Zhang, G.; Wen, M.; Wang, S.; et al. Insights into thermal reduction of the oxidized graphite from the electro-oxidation processing of nuclear graphite matrix. RSC Adv. 2018, 8, 567–579. https://doi.org/10.1039/C7RA11578D.
- 89.Marin, D.; Marchesan, S. Carbon Graphitization: Towards Greener Alternatives to Develop Nanomaterials for Targeted Drug Delivery. Biomedicines 2022, 10, 1320. https://doi.org/10.3390/biomedicines10061320.
- 90.Li, J.; Cheng, T.; Wu, H.; et al. CO2-assisted synthesis of graphitic resin-based activated carbon for ultrahigh selective adsorption of VOCs under humid conditions. Fuel 2023, 353, 129157. https://doi.org/10.1016/j.fuel.2023.129157.
- 91.Islam, F.; Tahmasebi, A.; Wang, R.; et al. Structure of Coal-Derived Metal-Supported Few-Layer Graphene Composite Materials Synthesized Using a Microwave-Assisted Catalytic Graphitization Process. Nanomaterials 2021, 11, 1672. https://doi.org/10.3390/nano11071672.
- 92.Shi, Z.; Wang, Y.; Lu, M.; et al. Catalytic graphitization of engineered pyrolysis bio-oil for sustainable graphite and hydrogen Co-production. Renew. Energy 2026, 256, 124149. https://doi.org/10.1016/j.renene.2025.124149.
- 93.Chen, X.; Deng, X.; Kim, N.Y.; et al. Graphitization of graphene oxide films under pressure. Carbon 2018, 132, 294–303.
- 94.Gentile, M.; Bellani, S.; Zappia, M.I.; et al. Hydrogen-Assisted Thermal Treatment of Electrode Materials for Electrochemical Double-Layer Capacitors. ACS Appl. Mater. Interfaces 2024, 16, 13706–13718. https://doi.org/10.1021/acsami.3c18629.
- 95.Zhao, J.G.; Li, F.Y.; Jin, C.Q. Graphitization of activated carbon under high pressures and high temperatures. Solid State Commun. 2009, 149, 818–821. https://doi.org/10.1016/j.ssc.2008.12.027.
- 96.Oluwole, O.S.; Jovanović; P; Mohonta, S.C.; et al. Low-temperature graphitization by amine-assisted combustion of graphite oxide. NPJ 2d Mater. Appl. 2025, 9, 52. https://doi.org/10.1038/s41699-025-00572-2.
- 97.Padwal, C.; Wang, X.; Pham, H.D.; et al. Efficient and swift heating technique for crafting highly graphitized carbon and crystalline silicon (Si@GC) composite anodes for lithium-ion batteries. Battery Energy 2024, 3, 20240025. https://doi.org/10.1002/bte2.20240025.
- 98.Lazareva, I.; Koval, Y.; Alam, M.; et al. Graphitization of polymer surfaces by low-energy ion irradiation. Appl. Phys. Lett. 2007, 90, 262108. https://doi.org/10.1063/1.2752738.
- 99.Antonelou, A.; Sygellou, L.; Vrettos, K.; et al. Efficient defect healing and ultralow sheet resistance of laser-assisted reduced graphene oxide at ambient conditions. Carbon 2018, 139, 492–499. https://doi.org/10.1016/j.carbon.2018.07.012.
- 100.Claro, P.I.C.; Pinheiro, T.; Silvestre, S.L.; et al. Sustainable carbon sources for green laser-induced graphene: A perspective on fundamental principles, applications, and challenges. Appl. Phys. Rev. 2022, 9, 041305. https://doi.org/10.1063/5.0100785.
- 101.Baghel, P.; Sakhiya, A.K.; Kaushal, P. Ultrafast growth of carbon nanotubes using microwave irradiation: Characterization and its potential applications. Heliyon 2022, 8, e10943. https://doi.org/10.1016/j.heliyon.2022.e10943.
- 102.Jones, L.; Goffin, N.; Ouyang, J.; et al. Laser specific energy consumption: How do laser systems compare to other manufacturing processes? J. Laser Appl. 2022, 34, 42029. https://doi.org/10.2351/7.0000790.
- 103.Olejnik, A.; Polaczek, K.; Szkodo, M.; et al. Laser-Induced Graphitization of Polydopamine on Titania Nanotubes. ACS Appl. Mater. Interfaces 2023, 15, 52921–52938. https://doi.org/10.1021/acsami.3c11580.
- 104.Kim, J.; Son, S.; Choe, M.; et al. In situ TEM investigation of nickel catalytic graphitization. Mater. Today Adv. 2024, 22, 100494. https://doi.org/10.1016/j.mtadv.2024.100494.
- 105.Shyam Kumar, C.N.; Chakravadhanula, V.S.K.; Riaz, A.; et al. Understanding Graphitization and Growth of free-standing Nanocrystalline Graphene using In Situ Transmission Electron Microscopy. Nanoscale 2017, 9, 12835–12842. https://doi.org/10.1039/C7NR03276E.
- 106.Zhang, F.; Liu, W. Recent progress of operando transmission electron microscopy in heterogeneous catalysis. Microstructures 2024, 4, 202404. https://doi.org/10.20517/microstructures.2024.03.
- 107.Schito, A.; Muirhead, D.K.; Parnell, J. Towards a kerogen-to-graphite kinetic model by means of Raman spectroscopy. Earth-Sci. Rev. 2023, 237, 104292. https://doi.org/10.1016/j.earscirev.2022.104292.
- 108.Gao, Y.; Zou, C.; She, Y.; et al. Analysis of Structural Heterogeneity in Low-Rank Coal and Its Pyrolyzed Char Using Multi-Point Scanning Micro-Raman Spectroscopy. Molecules 2024, 29, 2361. https://doi.org/10.3390/molecules29102361.
- 109.Zerda, T.; Gruber, T. Raman Study of Kinetics of Graphitization of Carbon Blacks. Rubber Chem. Technol. 2000, 73, 284–292. https://doi.org/10.5254/1.3547591.
- 110.Huali, W.; Ruchi, G.; Allen, C.; et al. Comparative Analysis of Microstructure and Reactive Sites for Nuclear Graphite IG-110 and Graphite Matrix A3. J. Nucl. Mater. 2020, 528, 151802.
- 111.Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; et al. Renewable and sustainable clean energy development and impact on social, economic, and environmental health. Energy Nexus 2022, 7, 100118. https://doi.org/10.1016/j.nexus.2022.100118.
- 112.Kim, T.; Lee, J.; Lee, K.-H. Full graphitization of amorphous carbon by microwave heating. RSC Adv. 2016, 6, 24667–24674. https://doi.org/10.1039/C6RA01989G.
- 113.Islam, F.; Wang, J.; Tahmasebi, A.; et al. Microwave-Assisted Coal-Derived Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries. Materials 2021, 14, 6468. https://doi.org/10.3390/ma14216468.
- 114.Liang, C.; Chen, Y.; Wu, M.; et al. Green synthesis of graphite from CO2 without graphitization process of amorphous carbon. Nat. Commun. 2021, 12, 119. https://doi.org/10.1038/s41467-020-20380-0.
- 115.Shi, Z.; Wang, S.; Jin, Y.; et al. Establishment of green graphite industry: Graphite from biomass and its various applications. SusMat 2023, 3, 402–415. https://doi.org/10.1002/sus2.139.
- 116.Sruthy, S.; Grimm, A.; Paul, M.; et al. Low-temperature Highly Graphitized Porous Biomass-based Carbon as an Efficient and Stable Electrode for Lithium-ion Batteries and Supercapacitors. Chem. Eng. J. Adv. 2025, 22, 100762. https://doi.org/10.1016/j.ceja.2025.100762.
- 117.Zhao, H.; Wu, H.; Rong, T.; et al. Green and efficient graphitization of biomass waste empowered by molten salt electrolysis: Mechanistic exploration and energy storage applications dual-driven by experiments and simulations. J. Mater. Chem. A 2025, 13, 3777–3790. https://doi.org/10.1039/D4TA07890J.
- 118.Li, K.; Liu, Q.; Cheng, H.; et al. Classification and carbon structural transformation from anthracite to natural coaly graphite by XRD, Raman spectroscopy, and HRTEM. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119286.
- 119.Pardanaud, C.; Cartry, G.; Lajaunie, L.; et al. Investigating the possible origin of Raman bands in defective sp2/sp3 carbons below 900 cm−1: Phonon density of states or double resonance mechanism at play? C 2019, 5, 79.
- 120.Li, G.; Zhang, H.; Han, Y. Applications of transmission electron microscopy in phase engineering of nanomaterials. Chem. Rev. 2023, 123, 10728–10749.
- 121.Su, Y.F.; Park, J.G.; Koo, A.; et al. Characterization at atomic resolution of carbon nanotube/resin interface in nanocomposites by mapping sp2-bonding states using electron energy-loss spectroscopy. Microsc. Microanal. 2016, 22, 666–672.
- 122.Vander Wal, R.L.; Bryg, V.M.; Hays, M.D. XPS analysis of combustion aerosols for chemical composition, surface chemistry, and carbon chemical state. Anal. Chem. 2011, 83, 1924–1930.
- 123.Seki, S.; Paitandi, R.P.; Choi, W.; et al. Electron transport over 2D molecular materials and assemblies. Acc. Chem. Res. 2024, 57, 2665–2677.
- 124.Munir, K.S.; Wen, C. Deterioration of the strong sp2 carbon network in carbon nanotubes during the mechanical dispersion processing—A review. Crit. Rev. Solid State Mater. Sci. 2016, 41, 347–366.
- 125.Mennani, M.; Ait Benhamou, A.; Mekkaoui, A.A.; et al. Probing the evolution in catalytic graphitization of biomass-based materials for enduring energetic applications. J. Mater. Chem. A 2024, 12, 6797–6825.
- 126.Li, N.; Li, X.; Wang, T.; et al. In situ transmission electron microscopy characterization and manipulation of the morphology, composition and phase evolution of nanomaterials under microenvironmental conditions. Chem. Sci. 2025, 16, 9604–9637.
- 127.Li, J.; Qin, Y.; Chen, Y.; et al. Structural characteristics and evolution of meta-anthracite to coaly graphite: A quantitative investigation using X-ray diffraction, Raman spectroscopy, and high-resolution transmission electron microscopy. Fuel 2023, 333, 126334.
- 128.Xue, B.; Ye, J.; Zhang, J. Highly conductive Poly (L-lactic acid) composites obtained via in situ expansion of graphite. J. Polym. Res. 2015, 22, 112.
- 129.Shi, Z.; Jin, Y.; Han, T.; et al. Bio-based anode material production for lithium–ion batteries through catalytic graphitization of biochar: The deployment of hybrid catalysts. Sci. Rep. 2024, 14, 3966. https://doi.org/10.1038/s41598-024-54509-8.
- 130.Guan, L.; Li, D.; Ji, S.; et al. Structural Regulation and Performance Enhancement of Carbon-Based Supercapacitors: Insights into Electrode Material Engineering. Materials 2025, 18, 456. https://doi.org/10.3390/ma18020456.
- 131.Hao, J.; Li, J.; Shi, X.; et al. Changes in microstructure and mechanical properties of the carbon fiber and their effects on C/SiC composites. Mater. Charact. 2022, 193, 112334. https://doi.org/10.1016/j.matchar.2022.112334.
- 132.Gao, Z.; Zhu, J.; Rajabpour, S.; et al. Graphene reinforced carbon fibers. Sci. Adv. 2025, 6, eaaz4191. https://doi.org/10.1126/sciadv.aaz4191.
- 133.Li, R.; Hu, J.; Li, Y.; et al. Graphene-Based, Flexible, Wearable Piezoresistive Sensors with High Sensitivity for Tiny Pressure Detection. Sensors 2025, 25, 423. https://doi.org/10.3390/s25020423.
- 134.Rzeźniczak, P.; Skrzetuska, E.; Venkataraman, M.; et al. Influence of the Ozonation Process on Expanded Graphite for Textile Gas Sensors. Sensors 2025, 25, 5328. https://doi.org/10.3390/s25175328.
- 135.Roselin, L.S.; Juang, R.S.; Hsieh, C.T.; et al. Recent advances and perspectives of carbon-based nanostructures as anode materials for Li-ion batteries. Materials 2019, 12, 1229.
- 136.Ávila Vázquez, V.; Enciso Hernández, E.A.; Kamaraj, S.K.; et al. Use of activated carbon and camphor carbon as cathode and clay cup as proton exchange membrane in a microbial fuel cell for the bioenergy production from crude glycerol biodegradation. J. Environ. Sci. Health Part A 2022, 57, 947–957.
- 137.Wang, G.; Yu, M.; Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443.
- 138.He, J.; Zhang, D.; Wang, Y.; et al. Biomass-derived porous carbons with tailored graphitization degree and pore size distribution for supercapacitors with ultra-high rate capability. Appl. Surf. Sci. 2020, 515, 146020.
- 139.Xia, Y.; Yang, Z.; Zhu, Y. Porous carbon-based materials for hydrogen storage: Advancement and challenges. J. Mater. Chem. A 2013, 1, 9365–9381.
- 140.Liu, J.; Huang, L.; Wang, H.; et al. The origin, characterization, and precise design and regulation of diverse hard carbon structures for targeted applications in lithium-/sodium-/potassium-ion batteries. Electrochem. Energy Rev. 2024, 7, 34.
- 141.Shao, Y.; Hourdin, L.; Sanchez, J.-Y.; et al. Fluorinated materials in electrochemical storage and conversion devices: assessment of advantages and disadvantages. C. R. Chimie 2025, 28, 523–541.
- 142.Hecht, D.S.; Hu, L.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513.
- 143.Xu, J.; Wang, Y.; Hu, S. Nanocomposites of graphene and graphene oxides: Synthesis, molecular functionalization and application in electrochemical sensors and biosensors: A review. Microchim. Acta 2017, 184, 1–44.
- 144.Raagulan, K.; Kim, B.M.; Chai, K.Y. Recent advancement of electromagnetic interference (EMI) shielding of two dimensional (2D) MXene and graphene aerogel composites. Nanomaterials 2020, 10, 702.
- 145.Fredj, Z.; Sawan, M. Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends. Biosensors 2023, 13, 211.
- 146.Ma, Z.; Wang, W.; Xiong, Y.; et al. Carbon Micro/Nano Machining toward Miniaturized Device: Structural Engineering, Large-Scale Fabrication, and Performance Optimization. Small 2024, 21, 2400179.
- 147.Sayam, A.; Rahman, A.M.; Rahman, M.S.; et al. A review on carbon fiber-reinforced hierarchical composites: Mechanical performance, manufacturing process, structural applications and allied challenges. Carbon Lett. 2022, 32, 1173–1205.
- 148.Ouyang, J.H.; Li, Y.F.; Zhang, Y.Z.; et al. High-temperature solid lubricants and self-lubricating composites: A critical review. Lubricants 2022, 10, 177.
- 149.Hassan, S.; Nadeem, A.Y.; Qaiser, H.; et al. A review of carbon-based materials and their coating techniques for biomedical implants applications. Carbon Lett. 2023, 33, 1171–1188.
- 150.Liu, Y.; Yang, J.; Wang, M.; et al. Recent developments in interface engineering strategies for stabilizing sodium metal anodes. Cell Rep. Phys. Sci. 2024, 5.
- 151.Zhou, X.; Qiao, J.; Yang, L.; et al. A review of graphene-based nanostructural materials for both catalyst supports and metal-free catalysts in PEM fuel cell oxygen reduction reactions. Adv. Energy Mater. 2014, 4, 1301523.
- 152.Gopinath, K.P.; Vo, D.V.; Gnana Prakash, D.; et al. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582.
- 153.Li, P.; Galek, P.; Grothe, J.; et al. Carbon-based iontronics–current state and future perspectives. Chem. Sci. 2025, 16, 7130–7154.
- 154.Alfieri, A.; Anantharaman, S.B.; Zhang, H.; et al. Nanomaterials for quantum information science and engineering. Adv. Mater. 2023, 35, 2109621.
- 155.Speranza, G. The role of functionalization in the applications of carbon materials: An overview. C 2019, 5, 84.
- 156.Sharma, S.; Basu, S.; Shetti, N.P.; et al. Versatile graphitized carbon nanofibers in energy applications. ACS Sustain. Chem. Eng. 2022, 10, 1334–1360.
- 157.Sikder, S.; Toha, M.; Rahman, M.M. Environmental Sustainability and Future Challenges of Waste-Derived Carbon Nanomaterials. In Waste Derived Carbon Nanomaterials; American Chemical Society: Washington, DC, USA, 2025; Volume 1, pp. 309–330.
- 158.Zhou, T.; Wu, X.; Liu, S.; et al. Biomass-derived catalytically active carbon materials for the air electrode of Zn-air batteries. ChemSusChem 2024, 17, e202301779.
- 159.Osman, A.I.; Nasr, M.; Mohamed, A.R.; et al. Life cycle assessment of hydrogen production, storage, and utilization toward sustainability. Wiley Interdiscip. Rev. Energy Environ. 2024, 13, e526.
- 160.Majumder, A.; Ray, B.C. Energy Efficiency and Sustainability. In Sinter Plants: Evolution, Challenges, and Future Perspectives; Springer Nature: Singapore, 2025; pp. 135–169
How to Cite
Montiel-García, A.; Srinivasan, M. K.; Aguilar, I. A.; Apollon, W.; Thirumurugan, A.; Kamaraj, S.-K. An Overview of the Transition from Amorphous Carbon to an Ordered Graphitic Crystalline Plane for Applications. Materials and Sustainability 2025, 1 (3), 12. https://doi.org/10.53941/matsus.2025.100012.
RIS
BibTex
Copyright & License

Copyright (c) 2025 by the authors.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Contents
References


